Microtubule motor proteins move across the microtubule, transporting cellular cargo within the cell. Different classes of microtubule motor protein provide motion through their interaction with tubulin subunits.
 Credit: Kateryna Kon/Shutterstock.com
Credit: Kateryna Kon/Shutterstock.com
Microtubule motor proteins can be either plus end motors or minus end motors depending on the direction of movement. Mitosis requires the trafficking of proteins along microtubules to produce two daughter cells with the same number of chromosomes as the parent cell. Motor proteins therefore have important roles in protein transport along the microtubules of the mitotic spindle during mitosis.
Kinesin motor proteins
There are two major classes of motor protein associated with movement along microtubules: the kinesins and dyneins. Both classes of microtubule motor protein display ATPase activity, with the energy required for moving proteins across the microtubule derived from the hydrolysis of ATP.
Kinesins have a wide range of functions including transporting structures such as chromosomes and regulating microtubule dynamics. The movement of kinesins across microtubules mostly occurs in the direction of the plus end, meaning cellular cargo is transported from the center of the cell to the periphery.
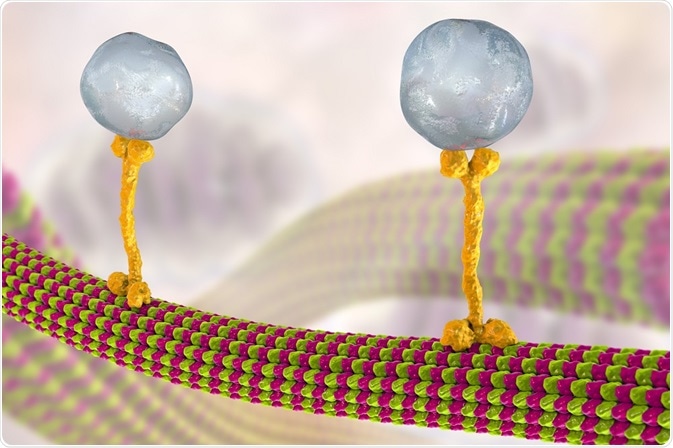
Kinesin structures vary, but all contain two globular heads formed from heavy chains that make up the motor domain, with separate binding sites for ATP and the microtubule. The stalks of two kinesin heavy chains produce a protein dimer that binds two light chains where most transported cargo attaches.
Kinesins move across microtubules by hydrolyzing one molecule of ATP at each step in a single direction to produce a “walking” motion. Kinesin motion is thought to be produced by either a hand-over-hand mechanism, where the globular heads alternate in step across the microtubule, or by an inchworm mechanism, in which one globular head leads and the other catches up. The results of recent studies are indicative of a hand-over-hand mechanism.
Dynein motor proteins
In contrast to most kinesins, dyneins move across microtubules towards the minus end. Cytoplasmic dynein has a similar structure to basic kinesins with two globular heads involved in the “walking” movement and light chains for attaching cargo.
The role of cytoplasmic dynein includes the transportation of cellular cargo necessary for cell function and to position certain organelles within the cell. Moreover, cytoplasmic dynein is vital to mitosis and the positioning of the mitotic spindle.
Cilia and flagella are projections on cells responsible for transporting materials past cells or for moving the entire cell. Axonemal dynein are the motor proteins that produce the beating motion within cilia and flagella. Each dynein molecule constructs a bridge between adjacent microtubules in the axoneme structure. The beating motion is formed from a siding movement between axoneme microtubules powered by dyneins.
 Credit: Kateryna Kon/Shutterstock.com
Credit: Kateryna Kon/Shutterstock.com
Microtubule motor proteins and mitosis
The rearrangement of microtubule subunits is fundamental for the formation of the mitotic spindle during mitosis. Cytoplasmic dynein is utilized in localizing the spindle poles and during the spindle checkpoint by removing checkpoint proteins from kinetochores.
The anaphase stage of mitosis is also driven by motor proteins. Chromosome movement is aided by the positioning of astral spindle microtubules through minus ended dynein motor proteins with cortical sliding of astral microtubules along stationary dynein possibly taking place during anaphase B.
Kinesins also have a role in mitosis by promoting proper spindle length and aiding microtubule movement during metaphase. For example, the Eg5 kinesin is involved in pushing the spindle poles in opposite directions by binding to antiparallel microtubules in the middle of the mitotic spindle. Other kinesins power movements towards the minus ends of microtubules and are utilized during anaphase along with dyneins.
Sources:
- Marx, A. et al. 2005. The structure of microtubule motor proteins, Advances in Protein Chemistry, 71, pp. 299-344.
- Ramaiya, A. et al. 2017. Kinesin rotates unidirectionally and generates torque while walking on microtubules, PNAS, 114, pp. 10894-10899.
- Lodish, H.., Berk, A., Zipursky, S.L. et al. 2000. Molecular Cell Biology. 4th edition. New York: W. H. Freeman. Section 19.5, Microtubule Dynamics and Motor Proteins during Mitosis.
- Wordeman, L. 2010. How Kinesin Motor Proteins Drive Mitotic Spindle Function: Lessons from Molecular Assays, Seminars in Cell and Developmental Biology, 21, pp. 260-268.
- Fink, G. et al. 2006. Dynein-mediated pulling forces drive rapid mitotic spindle elongation in Ustilago maydis, The EMBO Journal, 25, pp. 4897-4908.
Further Reading
Last Updated: Jul 19, 2023