From skin tone to rare mutations, scientists uncover how your genes influence vitamin D levels, and why standard supplements may not work the same for everyone.
 Review: Genetic Variants Influencing Individual Vitamin D Status. Image Credit: Zerbor / Shutterstock
Review: Genetic Variants Influencing Individual Vitamin D Status. Image Credit: Zerbor / Shutterstock
In a recent study published in the journal Nutrients, researchers in Canada reviewed the genetic mechanisms contributing to individual variation in vitamin D (VD) levels in circulation.
Humans can efficiently synthesize VD, which requires ultraviolet B (UVB) radiation exposure in the form of sunlight. However, at southern and northern latitudes distal to the equator, sunlight and skin temperature are inadequate for the synthesis of cholecalciferol (VD3) for several months of the year. As such, this could result in VD deficiency and adverse health outcomes in the absence of dietary sources.
Studies suggest that calcidiol (a major VD metabolite) levels of 60 ng/ml may be associated with a lower risk of certain health conditions. However, the optimal VD status is debated, and proposed thresholds vary across studies and expert groups. Importantly, most thresholds are derived from European cohorts, and their applicability across ancestries remains debated. Besides dietary and seasonal factors, genetics contribute to individual variation in VD. Gene-by-environment interactions, including skin tone and latitude, may also influence these outcomes. As such, the present study reviewed the genetic variants influencing individual VD status.
VD synthesis, regulation, and metabolism
VD can be acquired from diet, supplements, and endogenous synthesis. It is typically consumed as VD3 from dietary supplements and animal-based foods and ergocalciferol (VD2) from plant-based foods. VD is endogenously produced in humans under ideal conditions. Skin exposure to UVB light leads to the conversion of 7-dehydrocholesterol (7-DHC) into pre-VD3, which, after thermal isomerization, produces VD3.
Moreover, UVB irradiation converts pre-VD3 to tachysterol and lumisterol isomers, and VD3 to inactive suprasterols. This allows for VD regulation in the skin and prevents toxicity. The presence of lipids enhances the absorption of VD. The secretion of gastric lipase is required to hydrolyze esterified VD for its absorption. Subsequently, pancreatic lipases and bile acids in the small intestine promote the formation of micelles that solubilize VD.
Following absorption, VD is packaged and transported to the portal vein or indirectly via the lymphatic system to the blood. In circulation, VD3 binds to transport proteins that carry it to the liver. VD-binding protein (DBP) accounts for most of VD transport (85%) due to high binding affinity, while albumin transports about 15% of VD, leaving around 0.4% free VD in circulation.
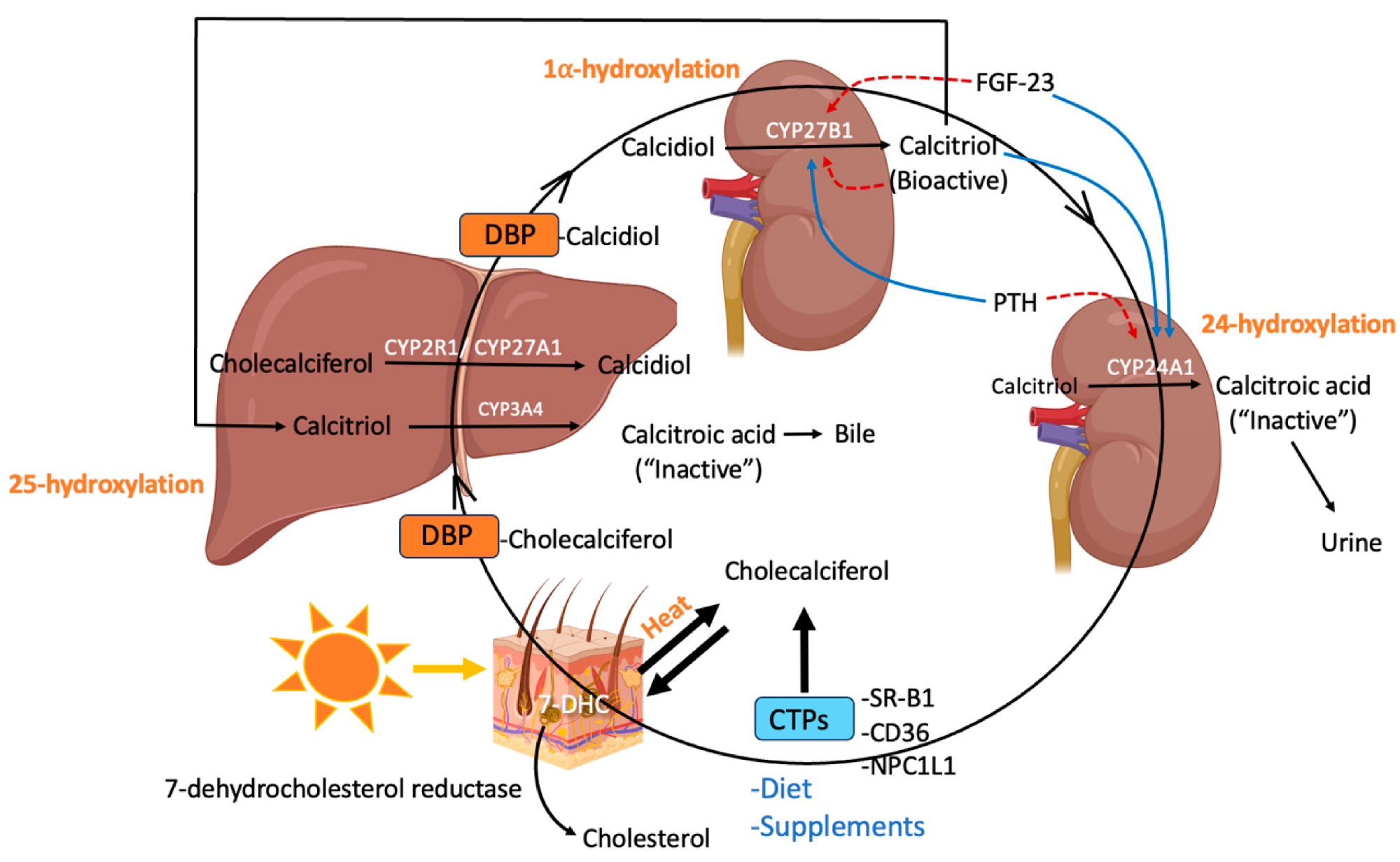 Synthesis, transport, and metabolism of vitamin D throughout the body (created using BioRender): 7-dehydrocholesterol (7-DHC), cholesterol-binding proteins (CTPs), scavenger receptor class B type 1 (SR-BI), Niemann-Pick C1-Like 1 (NPC1L1), vitamin D binding protein (DBP), fibroblast growth factor (FGF)-23 (FGF-23), parathyroid hormone (PTH).
Synthesis, transport, and metabolism of vitamin D throughout the body (created using BioRender): 7-dehydrocholesterol (7-DHC), cholesterol-binding proteins (CTPs), scavenger receptor class B type 1 (SR-BI), Niemann-Pick C1-Like 1 (NPC1L1), vitamin D binding protein (DBP), fibroblast growth factor (FGF)-23 (FGF-23), parathyroid hormone (PTH).
Genetic variants influencing VD status
Genome-wide association studies (GWASs) and candidate gene association studies (CGASs) have investigated polymorphisms in the VD receptor (VDR) gene associated with non-skeletal and skeletal health. Nevertheless, information on the association with genes related to VD synthesis, metabolism, and transport is lacking. GWASs have reported associations with single-nucleotide polymorphisms (SNPs) in 7-DHC reductase (DHCR7) and VD levels.
Several DHCR7 mutations that reduce its expression during fetal development are responsible for a rare autosomal recessive disease, Smith-Lemli-Opitz syndrome. Loss-of-function mutations in both DHCR7 alleles (biallelic frameshift mutations) result in impaired cholesterol biosynthesis. Common SNPs in DHCR7, such as rs12785878, are associated with lower serum VD and are more prevalent in northern populations, suggesting potential evolutionary adaptation to latitude. In the context of VD synthesis, lower functional DHCR7 levels would reduce 7-DHC-to-cholesterol conversion, increasing the availability of 7-DHC for VD synthesis.
The GC gene encodes DBP, the primary transporter of VD metabolites. Two missense SNPs define the common isoforms of GC: GC1s, GC2, and GC1f, which differ in VD binding affinity and DBP concentration. The GC1f allele has been associated with lower serum levels of calcidiol and increased risk of hypovitaminosis D in infants.
Although not as well-studied as GC, several candidate transport proteins (CTPs) have been proposed to influence VD absorption and distribution. Cholesterol-transport proteins (CTPs) transport VD, vitamin K, α-tocopherol, and carotenoids, among others, throughout the body. A study found that mice deficient in a CTP, scavenger receptor class B type 1 (SCARB1), had elevated levels of deuterated VD3 in serum, heart, adipose tissue, and kidney, but reduced levels of deuterated calcidiol in kidney, serum, and liver. While direct associations between human CTP variants and serum calcidiol are lacking, established effects on the transport of other fat-soluble vitamins indicate their involvement is plausible. Emerging research also highlights similar transport roles for CD36 and NPC1L1, though more evidence is needed to confirm their contribution to VD status. Overall, while the role of CTPs in VD regulation remains plausible, further validation in human studies is required.
Cytochrome P450 family 2 subfamily R member 1 (CYP2R1) is responsible for the hydroxylation of VD3 to calcidiol. Various GWASs and CGASs have uncovered associations between SNPs in CYP2R1 and VD status. A study reported that 21 non-synonymous polymorphisms in CYP2R1 decreased CYP2R1 activity, while two SNPs increased activity. A risk allele, rs10741657, in CYP2R1 was reported to be associated with a higher likelihood of VD insufficiency.
Further, CYP27B1 accounts for the hydroxylation of calcidiol to calcitriol. SNPs in CYP27B1 have been associated with circulating VD levels. For instance, rs4646536, an intronic SNP in CYP27B1, has been associated with VD deficiency risk. Besides, loss-of-function mutations in CYP27B1 cause VD-dependent rickets type 1A (VDDR1A). VDDR1A patients with biallelic loss-of-function mutations in CYP27B1 have undetectable or low calcitriol, requiring life-long calcitriol supplementation.
CYP24A1 is responsible for calcitriol inactivation. Various SNPs in CYP24A1 have been associated with VD concentrations. A case-control study reported that specific CYP24A1 SNP genotypes (intronic rs2585428 and rs4809960) were associated with VD deficiency risk. Another study found that a CYP24A1 SNP, rs172167070, was associated with calcidiol levels and VD responder status in a pediatric population with cystic fibrosis. Rare CYP24A1 loss-of-function mutations may underlie hypersensitivity to VD supplementation, suggesting a role for genetic testing in specific clinical scenarios.
Additionally, the review discusses two other genes not commonly addressed in prior studies: CYP11A1, which generates alternative bioactive vitamin D metabolites, especially in the skin. CYP3A4, a hepatic enzyme involved in the inactivation of both calcidiol and calcitriol, where a rare gain-of-function mutation was linked to rickets in children.
Concluding remarks
VD status is a polygenic trait affected by polymorphisms in genes regulating its synthesis, metabolism, and transport, and rare mutations that can alter VD metabolism. Variants in the vitamin D receptor (VDR) may also indirectly influence circulating levels via gene regulation mechanisms. These genetic factors, as well as seasonal variation, mean that sufficient VD levels cannot always be attained through standard dietary recommendations. Understanding the cumulative effects of the environment, genetics, and gene-environment interactions is necessary to develop precise recommendations.
The review also highlights clinical implications. Vitamin D genetic risk scores (GRSs) may eventually help identify individuals at risk of deficiency or toxicity, enabling more tailored supplementation strategies. However, these tools require further validation in diverse populations. In clinical practice, a phenotype-driven approach remains most feasible, though emerging methods, such as high-dose cholecalciferol challenges, could assess individual bioavailability and metabolic response.
Journal reference:
- Karrow NA, Leuschner SE, Shandilya UK, Mallard BA, Wagter-Lesperance L, Bridle BW (2025). Genetic Variants Influencing Individual Vitamin D Status. Nutrients, 17(16), 2673. DOI: 10.3390/nu17162673, https://www.mdpi.com/2072-6643/17/16/2673