The human brain, with its 100 billion neurons that control every thought, word, and action, is the most complex and delicate organ in the body. Because it needs extra protection from toxins and other harmful substances, the blood vessels that supply the brain with oxygen and nutrients are highly selective about which molecules can cross from the blood into the brain and vice versa. These blood vessels and their unique network of supporting pericyte and astrocyte cells comprise the blood-brain barrier (BBB).
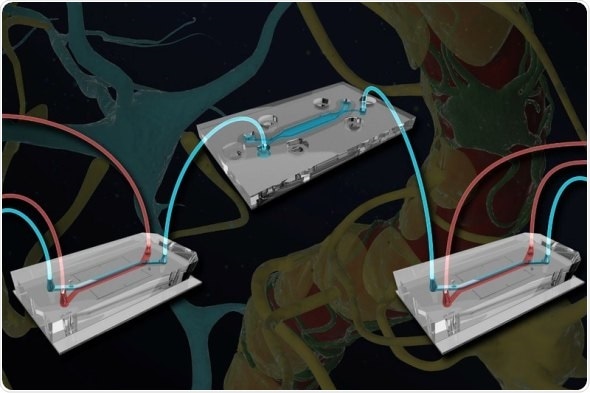
One Brain Chip (top) containing neurons and astrocytes is connected via microfluidic channels to two blood-brain barrier (BBB) chips containing endothelial cells and their supporting astrocytes and pericytes. The researchers were able to trace the flow of molecules from the vasculature across the BBB and into the brain, and found that substances produced by the endothelial cells help maintain neuronal function. Credit: Wyss Institute at Harvard University
Figuring out exactly how the cells of the BBB and the brain influence each other has been a major challenge in neuroscience. Now, researchers at the Wyss Institute for Biologically Inspired Engineering have created a “just right” model of the BBB-brain interface using microfluidically linked Organ Chips that allow an unprecedented look into how the brain’s vasculature influences and regulates its metabolic function. The research is reported in Nature Biotechnology.
“Most of today’s research on Organ Chips is focused on trying to increase the complexity of cell types on each chip, but we realized that the brain is already so complex that we couldn’t analyze it on one chip, so we did the opposite and divided one organ onto multiple chips,” said first author Ben Maoz, Ph.D., a former Technology Development Fellow at the Wyss Institute who is currently an Assistant Professor at Tel Aviv University, Israel. Joining Maoz as co-first authors of the paper are former Wyss Institute colleagues Anna Herland, Ph.D., who is now an Associate Professor at the Royal Institute of Technology and the Karolinska Institute in Stockholm, Sweden; and Edward FitzGerald, Ph.D., currently an Industrial Marie Skłodowska-Curie Fellow at Beactica AB and Uppsala University, Uppsala, Sweden.
The BBB-Brain Chip system consists of three chips: one “influx” BBB Chip, a Brain Chip, and a second “efflux” BBB Chip, all connected with microfluidic channels to allow the exchange of chemicals and other substances between them. The BBB Chip has one channel lined with endothelial blood vessel cells separated by a porous membrane from a parallel channel containing supportive pericytes and astrocytes in cerebrospinal fluid. The Brain Chip has similar parallel channels that are separated by a semipermeable membrane, one of which contains human brain neurons and their supporting astrocytes to mimic brain tissue. The three chips’ channels are connected together in series, creating a fully linked system in which substances can diffuse from the first BBB chip’s vascular channel into its cerebrospinal channel, enter the Brain Chip’s neuronal cell compartment, flow back into the cerebrospinal channel, and ultimately diffuse out into the second BBB Chip’s vascular channel, as happens in vivo.
The team cultured human cells in the linked BBB-Brain Chips and exposed them to methamphetamine, which is known to disrupt the junctions between the cells of the BBB in vivo and cause it to “leak.” When meth was flowed through the blood vessel channel of the BBB Chip, it compromised the junctions of the BBB’s vascular endothelial cells and allowed the passage of molecules that normally wouldn’t be able to cross the BBB into the Brain Chip. This experiment confirmed that the model worked, and established that it could be used in research to better understand drugs’ effects on the human brain and develop treatments.
The scientists also discovered that the proteins expressed by the cells on linked BBB and Brain Chips were different from those expressed by cells on unlinked chips. For example, cells in all of the linked chips expressed higher levels of metabolism-associated proteins and lower levels of proteins involved in proliferation and migration than cells in unlinked chips, suggesting that the BBB and the brain do in fact help each other maintain proper function.
“Blood vessels are frequently thought to just be a barrier or a transporter of chemicals. But when we looked at the linked BBB-Brain Chips, we noticed that there seemed to be some crosstalk between the endothelial cells and the neurons,” explained Herland. “We also know from studies of long-term meth abusers that this drug affects the brain’s metabolism, so we started to dig deeper to see if we could characterize the metabolic link between the BBB and the brain.”
That scientific digging revealed that the chemicals secreted by the cells on the uncoupled BBB Chip were largely related to neuron maintenance and protection, demonstrating that the molecules produced by the BBB provide chemical cues to neurons. The researchers also added
radioactive carbon-labeled glucose, pyruvate, or lactate as an energy supply to decoupled Brain Chips, and found that the production of both glutamine and the neurotransmitter GABA was lower in unlinked Brain Chips than in Brain Chips linked to the BBB. Intriguingly, this finding demonstrated that products of vascular endothelial cell metabolism become substrates for the production of neurotransmitters by neurons, suggesting that the health of our blood vessels could have a direct impact on mind function.
“The big breakthrough here is that we have teased out communication networks between cells in a way that never could have been done with traditional brain research techniques. In vivo studies simply do not offer the granularity to determine how complex these metabolic networks function in heterogeneous cell populations within living tissues,” said corresponding author Kit Parker, Ph.D., a Core Faculty member of the Wyss Institute and the Tarr Family Professor of Bioengineering and Applied Physics at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS). “We are seeing here an unanticipated level of complexity that raises the bar in terms of what it will mean to successfully map the brain's connectome.”
“What’s really incredible is that we were able to do a highly multiplexed, massively parallel metabolomic analysis of many different chemicals produced by different cell types, all on these tiny chips,” said co-corresponding author and Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at SEAS. “We’re excited to push the limits of how complicated and sophisticated Organ Chips can be, and potentially use this decoupling approach to analyze how vascular endothelial cells contribute to the specialized functions of other organs as well.”