The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially thought to be a respiratory infection, but further research has revealed that the coronavirus can infect and damage multiple areas in the body. Previous work has shown that SARS-CoV-2 can bind to ACE2 receptors in the kidneys, heart, lungs, and more. The virus has also been shown to infect the brain as some people experience neurological complications during and after COVID-19 infection. However, the mechanism as to how the coronavirus infects the brain remains poorly understood.
A recent preclinical study led by Anja Kipar of the University of Zurich and the University of Liverpool found evidence of the virus infecting and eventually provoking cell death in microglia and immune cells — but not neurons. The viral-induced apoptosis in these brain cells may explain why some long-haulers experience ‘brain fog’ months after recovery.
The study “Viral neuroinvasion and neurotropism without neuronal damage in the hACE2 mouse model of COVID-19” is available as a preprint on the bioRxiv* server, while the article undergoes peer review.
How they did it
The researchers used the transgenic K18-hACE2 mice that have been verified as a reliable model for SARS-CoV-2 infection. The mice were placed intranasally infected with low or high SARS-CoV-2 doses followed by influenza A virus infection. The virus entered the brain seven days after intranasal administration.
Proposed pathway for viral entry into the brain
Because of the low intranasal dose, the brain was not entirely infected by SARS-CoV-2. The virus occupied the frontal brain regions, including the caudoputamen, the hippocampal area, and the thalamus/hypothalamus area. At low levels, the virus did not spread to the spinal cord. The researchers observed viral spread in the brain and spinal cord at higher doses, indicating infection is dose-dependent.
The virus likely enters through the olfactory bulb. The researchers made this deduction when they found brain areas infected with SARS-CoV-2 included the olfactory epithelium and neurons in the olfactory bulb. Neurons with secondary or tertiary connections to the olfactory bulb were also infected with SARS-CoV-2.
However, the team did note that the virus occupied brain areas with no direct connection to the olfactory bulb, suggesting multiple viral entries into the brain.
The front-to-back viral spread observed in the study suggests SARS-CoV-2 likely proceeds through a direct invasion of the brain starting at the front. The front of the brain has several cortical areas involved in memory, attention, and executive function — abilities that may become impaired during COVID-19 infection. The findings could also explain other COVID-19 related symptoms.
“In mild COVID-19, infection of the brain via the olfactory bulb and possible other neurogenic routes could occur. This would then lead to exclusive neuronal infection and only a mild inflammatory response. It might be this scenario that explains the loss of taste and smell and severe headaches reported in patients with mild disease.”
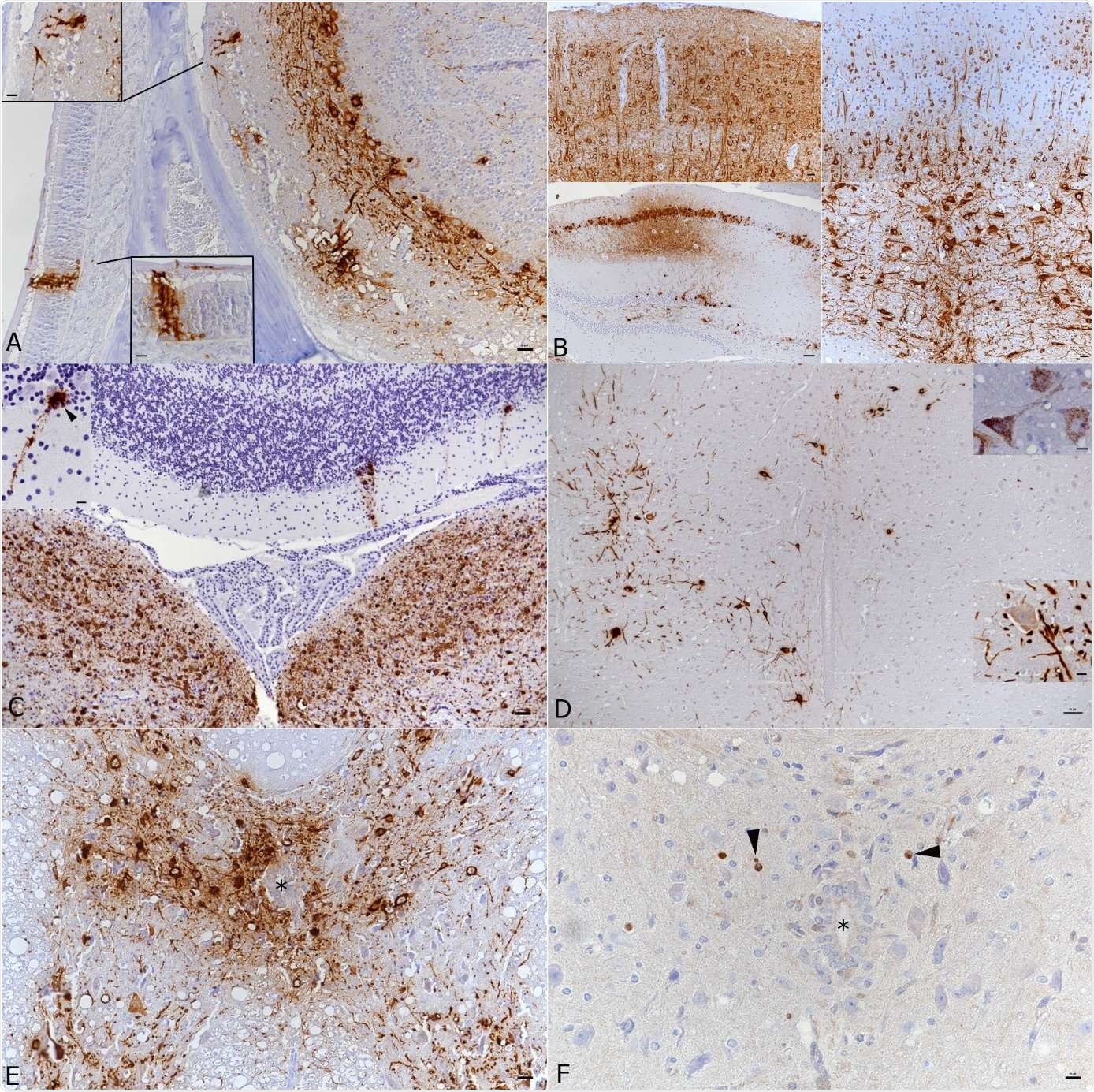
Brain and spinal cord, K18-hACE mice, day 7 post intranasal SARS-CoV-2 infection (104 PFU as single infection or 103 PFU after initial infection with IAV (102 PFU IAV strain A/X31) as double infection). A) Double infected animal, main olfactory epithelium (MOI), cribriform plate and olfactory bulb. Viral antigen is detected in olfactory neurons and basal cells of the MOI and in granule layer, inner and outer plexiform layer, mitral layer as well as glomerular layer of the olfactory bulb. Bar = 20 μm. B) Double infected animal, examples of viral antigen expression in different brain regions. Top: frontal cortex with viral antigen expression in almost all neurons = 20 μm. Bottom: patchy virus antigen expression in the hippocampus (CA1 and CA3; left; bar = 50 μm.) and strong expression in the medulla oblongata (right; bar = 20 μm.). C) Single infected animal, medulla oblongata and cerebellum. Viral RNA is abundantly expressed in neurons in the medulla oblongata (vestibular nuclei). The cerebellar cortex exhibits a few positive Purkinje cells (see also inset). Bar = 50 μm. D) Single infected animal, thoracic spinal cord. The grey matter exhibits numerous neurons that express viral antigen (large image and bottom inset) and viral RNA (top inset) in cell body and processes. Bar = 50 μm. E, F) Double infected animal, thoracic spinal cord. There is extensive viral antigen expression in neurons in the grey matter (E). Consecutive section showing scattered apoptotic (cleaved caspase 3 positive) glial cells (F, arrowheads), among intact neurons and in the absence of an inflammatory reaction. * - central canal. Bars = 20 μm. Immunohistochemistry and RNA-ISH, hematoxylin counterstain.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 infection in multiple brain cell types
SARS-CoV-2 infected neurons, and researchers observed an increase in viral protein in the cell’s cytoplasm. But the virus never caused neurons to die, which suggests either the virus provides no direct cytopathic effect on neurons or the dosage was too low to cause significant damage. There was no evidence of demyelination, which would shorten the speed of neurotransmission between cells or axonal damage.
There were instances of mild encephalitis in mice with the viral antigen, and the immune system induced it. This was dose-dependent again, with a higher viral dose increasing the severity of infection. The encephalitis was mainly driven by T cells and macrophages, and the researchers also observed increased neuronal inflammation in infected mice.
Neurons didn’t undergo cell death with the viral intrusion, but other cells —infiltrating lymphocytes, capillary endothelial cells, and macrophages/microglial cells were not as fortunate. Despite undergoing apoptosis, none of the cells were directly infected by the virus.
“This result is in line with recent findings in SARS-CoV-2 infected human brain organoids which provided evidence that infected neurons do not die but can promote the death of adjacent uninfected cells,” wrote the researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Seehusen F, et al. Viral neuroinvasion and neurotropism without neuronal damage in the hACE2 mouse model of COVID-19. medRxiv, 2021. doi: https://doi.org/10.1101/2021.04.16.440173, https://www.biorxiv.org/content/10.1101/2021.04.16.440173v1
- Peer reviewed and published scientific report.
Seehusen, Frauke, Jordan J. Clark, Parul Sharma, Eleanor G. Bentley, Adam Kirby, Krishanthi Subramaniam, Sabina Wunderlin-Giuliani, et al. 2022. “Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-HACE2 Mouse.” Viruses 14 (5): 1020. https://doi.org/10.3390/v14051020. https://www.mdpi.com/1999-4915/14/5/1020.