In this interview, NewsMedical speaks with Dr. Yuying Wang, Chief Technology Officer of Oncology from BGI Genomics, about colorectal cancer and the development and application of COLOTECT® 1.0, a non-invasive fecal DNA test for the detection of colorectal cancer and precancerous lesions.
1. What are the risk factors for colorectal cancer, and how have lifestyle changes contributed to increased incidence and mortality rates?
Colorectal cancer is a major contributor to cancer-related mortalities globally. According to GLOBOCAN 2020, it ranks third in incidence (1,931,590 cases) and second in mortality (935,173 cases) following lung cancer.1 The incidence and mortality rates of colorectal cancer have been increasing for several decades.
Most risk factors associated with colorectal cancer are related to lifestyle and age, such as the use of alcohol and tobacco, lack of physical exercise, a low-fiber diet, and increasing age. Other risk factors include a family history of colon cancer and a personal history of inflammatory intestinal conditions.
Global economic growth has brought about profound changes in lifestyles and increased lifespans, which have contributed to the surge in colorectal cancer incidence and mortality rates.
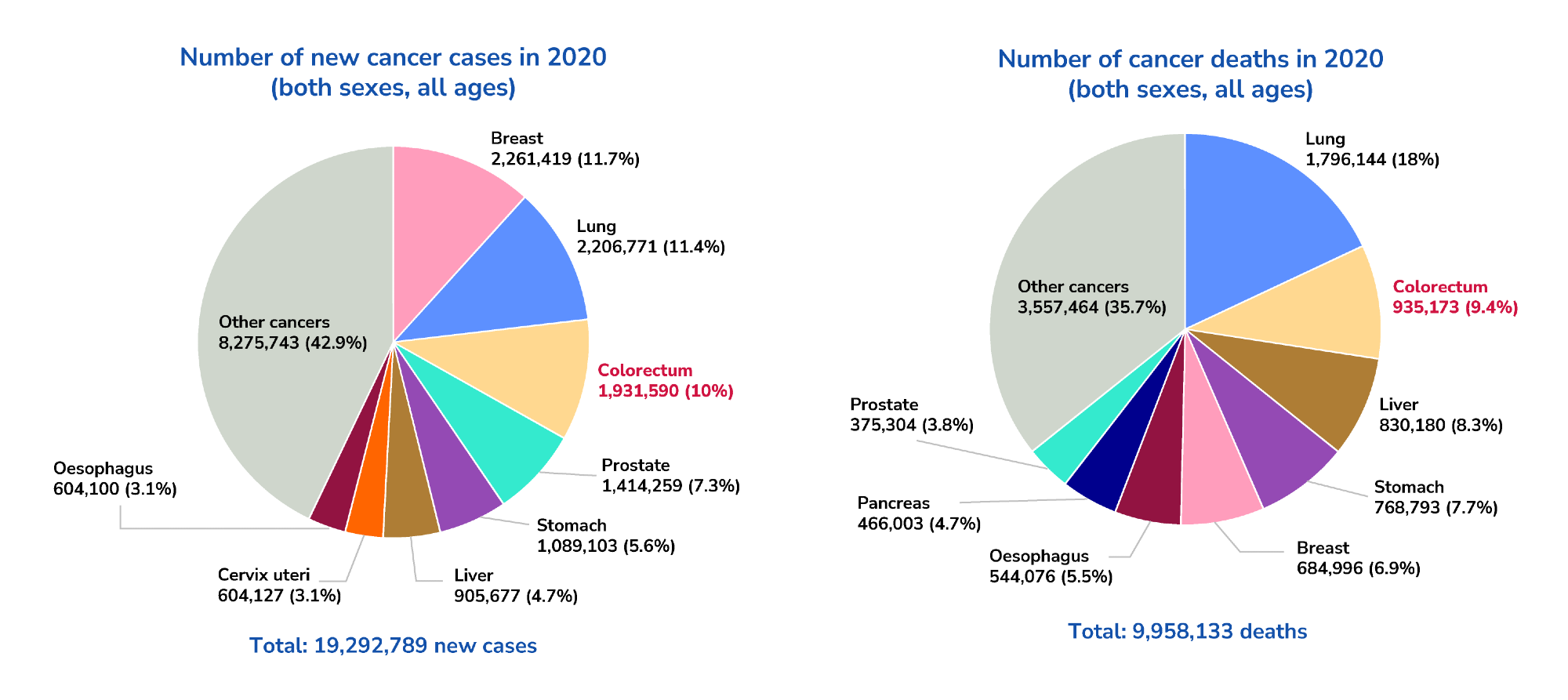
Image Credit: BGI Genomics
2. What is the current trend in the age-specific incidence of colorectal cancer?
Age is a significant factor in the increasing incidence of colorectal cancer. Studies and publications have demonstrated an escalating trend of colorectal cancer incidence with increasing age. The incidence of colorectal cancer generally rises sharply after 50 years of age,2 and an increasing trend is observed in individuals aged between 40 and 44, showing that early-onset colorectal cancer incidence has been rising at an alarming pace.3
While most European guidelines recommend average-risk population shall start colorectal cancer screening at age 50, there are now clinical guidelines, such as the American Cancer Society Colorectal Cancer Screening Guideline (2018), recommending colorectal cancer screening at 45 years old instead of 50 years old, because of the concerning increase in colorectal cancer incidence among younger individuals.
3. Why is early detection of colorectal cancer crucial for improving survival rate?
The progression of colorectal cancer development tends to be very slow. It can take up to 10 to 15 years to develop from normal epithelium to hyperplasia, to adenomatous polyps, and undergo malignant transformation to become colorectal cancer.
This extended window period offers a favorable opportunity for early detection and intervention, making it possible to detect the disease before it becomes malignant or progresses to later stages. Early detection of colorectal cancer, such as at stage I or II, allows a higher chance of curing the disease or finding better patient treatment options.
A cohort study indicates that removing adenomatous polyps of the colon and rectum can reduce the incidence of colorectal cancer cases by 76-90%.4 Statistics from the SEER Program of the National Cancer Institute also show that the five-year survival rate of early-stage colorectal cancer (stage I-II) can be as high as 90.6%.5 In contrast, the five-year survival rate drops significantly to 14.7% when diagnosed at stage IV.5
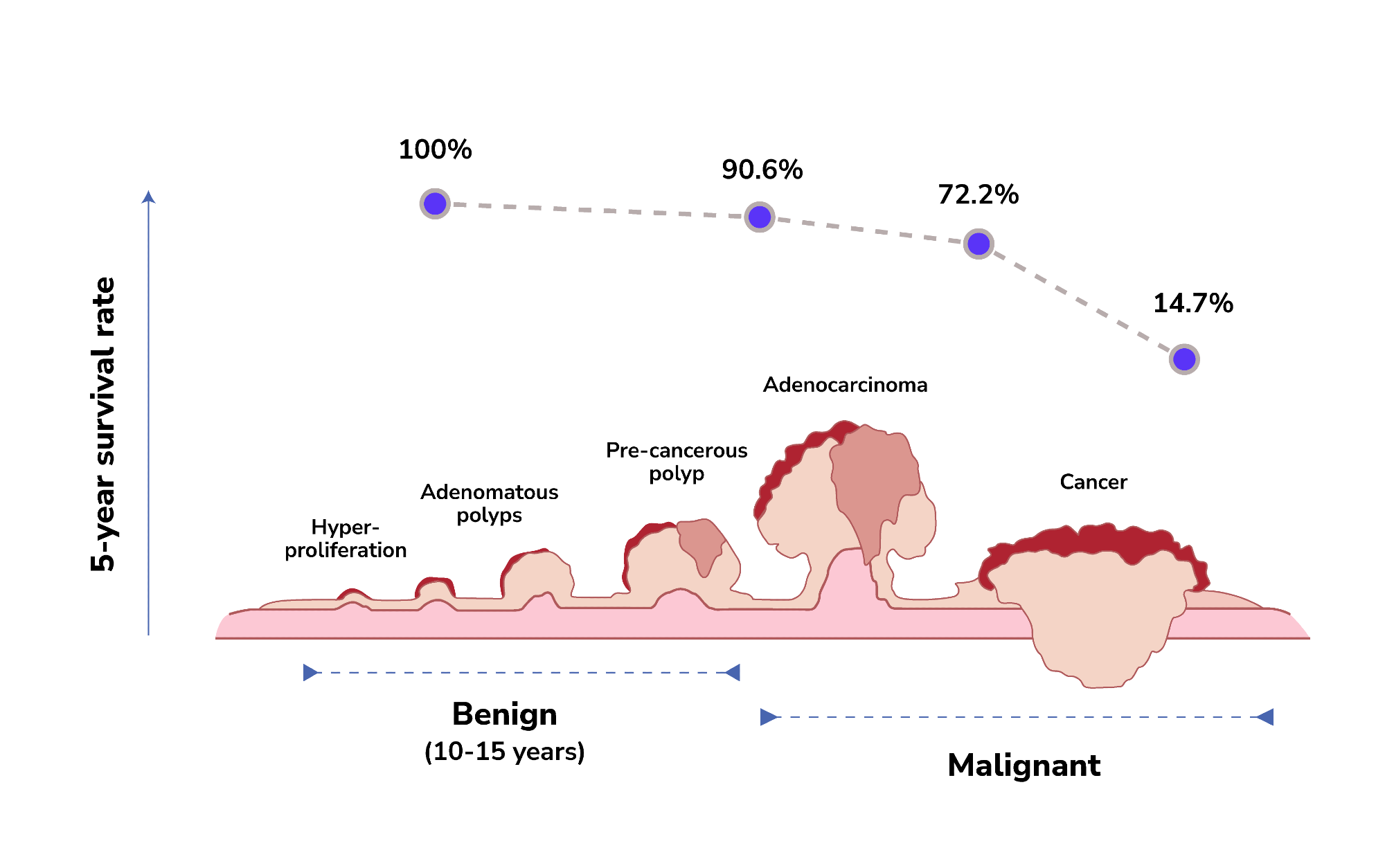
Image Credit: BGI Genomics
4. What are the possible limitations of colonoscopy? How can fecal DNA testing be used as an alternative for the early detection of colorectal cancer?
Although colonoscopy remains the gold standard tool for colorectal cancer screening, there are several limitations to its use in the general population.
Colonoscopy can be quite expensive in some areas of the world, and the procedure is invasive, often resulting in lower adherence due to the relatively lengthy and complicated preparation process. In addition, complications associated with anesthesia may occur.
Fecal DNA testing is a non-invasive strategy that can be used as an alternative option for detecting colorectal cancer pre-cancerous lesions.
The principle behind this test is that both normal colon epithelium and colorectal neoplasms shed cells into the intestinal lumen through normal cell turnover and apoptosis. These cells will move along the intestine with feces and can be collected with bowel movement. Fecal DNA can then be extracted to detect abnormal genomic signals.
DNA methylation is an ideal type of biomarker for cancer detection. It is an important form of epigenetic modification that is closely associated with cancer progression and can cause aberrant gene expression. Abnormal DNA methylation is an early event that occurs during carcinogenesis, making it a promising biomarker for the early detection of malignant tumors.
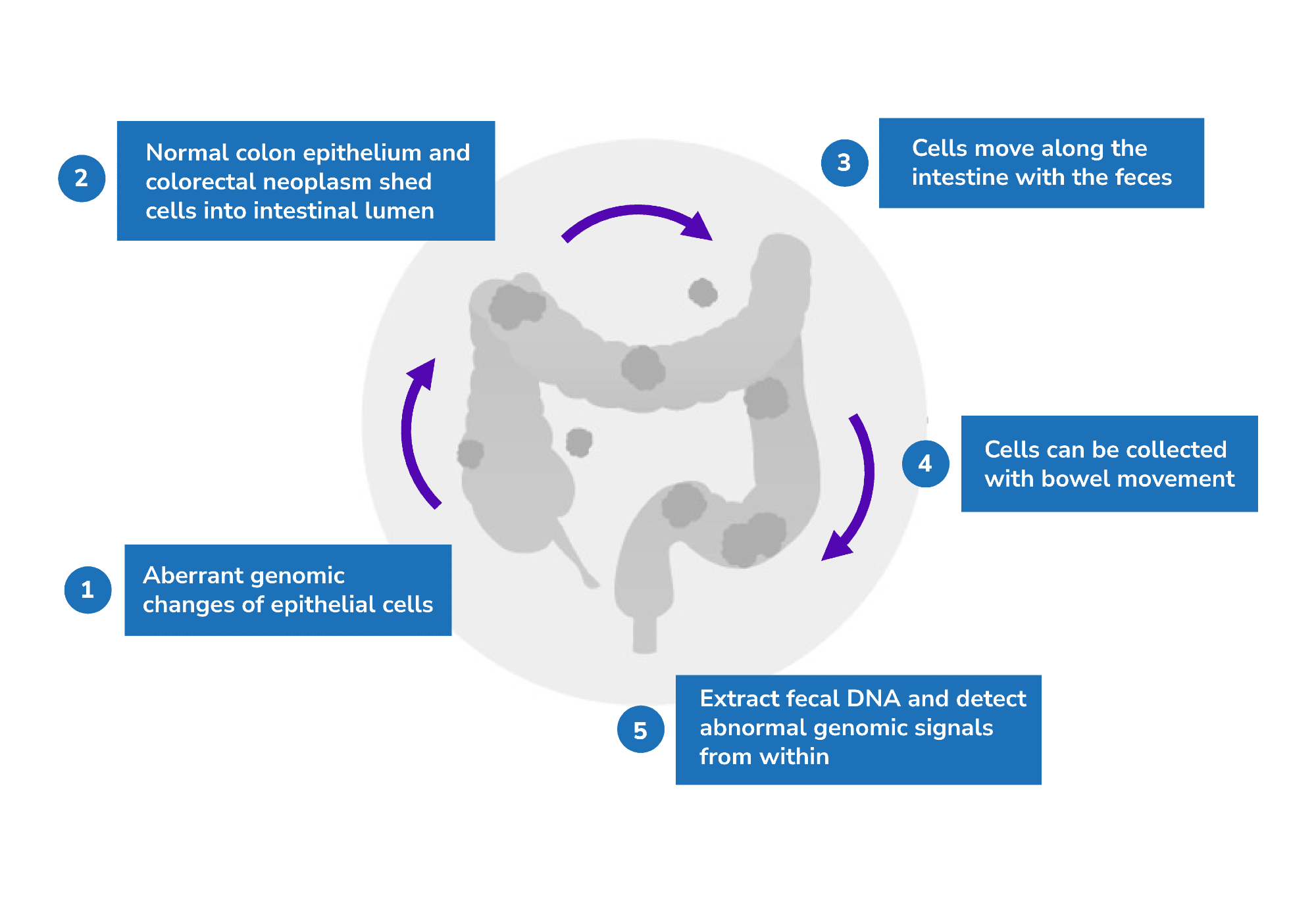
Image Credit: BGI Genomics
5. Can you explain the development process of BGI’s fecal DNA test, COLOTECT® 1.0?
COLOTECT® 1.0 is a non-invasive fecal DNA test that utilizes multiplex methylation-specific PCR technology to trace abnormal DNA-methylation biomarkers in colorectal cancer from fecal samples.
The development of COLOTECT® 1.0 involved whole-genome bisulfite sequencing (WGBS) of colorectal cancer tumor tissues and paired normal tissues to identify and discover colorectal cancer-associated methylation-specific gene markers.
We reviewed potential markers by querying public genomic databases, such as The Cancer Genome Atlas (TCGA) database. After that, specific candidate genes were identified, and primer and probe pairs were designed to formulate quantitative PCR (qPCR) assays.
These markers were then validated using clinical fecal samples collected from patients and healthy control subjects. A panel of three biomarkers, SDC2, ADHFE1, and PPP2R5C, were ultimately selected to formulate the COLOTECT® 1.0 assay.
6. How is the performance of COLOTECT® 1.0 for detecting colorectal cancer and advanced adenoma?
Clinical validation of the COLOTECT® 1.0 assay was conducted to determine its performance metrics in detecting colorectal cancer or pre-cancerous lesions.
Overall, its sensitivity for detecting colorectal cancers is 88%, and its specificity for identifying subjects without advanced colorectal neoplasm is 92%.6 Additionally, COLOTECT® 1.0 has a sensitivity rate of 46% for detecting advanced adenoma cases.6
The performance of COLOTECT® 1.0 was also compared to a fecal immunochemical test (FIT), which detects fecal occult blood instead of DNA methylation. COLOTECT® 1.0 exhibits better sensitivity for detecting both colorectal cancer and advanced adenoma.
This demonstrates that a multi-gene methylation marker panel can outperform FIT, offering better opportunities to detect early colorectal cancer and advanced adenoma cases.
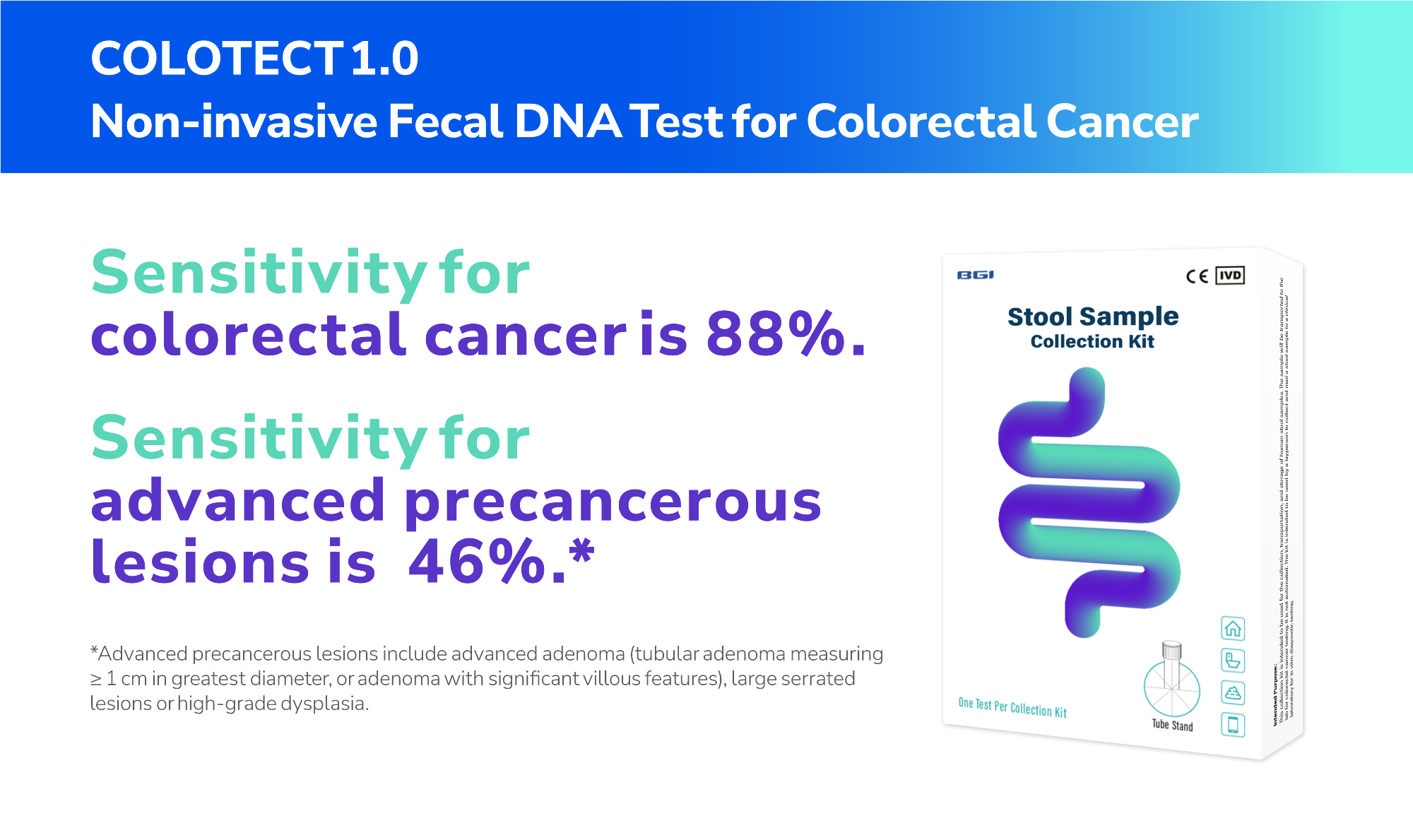
Image Credit: BGI Genomics
7. Can you share BGI’s experience using COLOTECT® 1.0 to conduct large-scale pilot population screening program?
BGI has conducted several large-scale prospective pilot studies across China to test the effectiveness of using COLOTECT® 1.0 for general population screening.
One such pilot study was conducted in Shandong Province in Eastern China, which recruited more than 18,000 average population participants between the years 2020 and 2021. The participants, aged between 40 and 74, self-sampled their stool specimens, and the COLOTECT® 1.0 assay was performed.
Results showed that close to 6% of participants tested positive, and nearly 500 of them underwent colonoscopy, resulting in an adherence rate of 47%, which is remarkable considering the poor adherence rate for colonoscopy in the general population. Among those who underwent colonoscopy, more than 300 abnormal cases were detected.
Dr. Yuying Wang
Dr. Yuying Wang is the Chief Technology Officer of Oncology at BGI Genomics. Dr. Wang has a Ph.D. in Cell Biology from the University of Illinois at Urbana-Champaign and did postdoctoral research at the University of Colorado School of Medicine.
Dr. Wang is committed and dedicated to research in cell biology and cancer genomics, and has extensive experience in the fields of tumor markers, cancer diagnosis and screening technology, high-throughput sequencing, and liquid biopsy.
About BGI Genomics 
BGI Genomics is the world's leading integrated solutions provider of precision medicine, now serving customers in more than 100 countries.
They provide academic institutions, pharmaceutical companies, healthcare providers, and other organizations with integrated genomic sequencing, proteomic services, clinical testing, and solutions across a broad range of applications.
They have more than 20 years of genomics experience helping customers and partners achieve their goals by delivering rapid, high-quality results using a broad array of cost-effective, cutting-edge technologies, including their own innovative DNBSEQ™ sequencing technology.
References
- International Agency for Research on Cancer (2020). GLOBOCAN 2020: new global cancer data, https://www.uicc.org/news/globocan-2020-new-global-cancer-data#:~:text=What%20is%20GLOBOCAN%3F,for%20all%20cancer%20sites%20combined, accessed Jan 2023
- Thrumurthy SG, Thrumurthy SS, Gilbert CE, Ross P, Haji A. Colorectal adenocarcinoma: risks, prevention, and diagnosis. BMJ. 2016;354:i3590. Published 2016 Jul 14. doi:10.1136/bmj.i3590
- Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7(3):262-274. doi:10.1016/S2468-1253(21)00426-X
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977-1981. doi:10.1056/NEJM199312303292701
- National Cancer Insititue (2000). SEER 18 2011-2017, All races, both sexes, https://seer.cancer.gov/statfacts/html/colorect.html, accessed Jan 2023
-
Fang, Y., Peng, J., Li, Z., Jiang, R., Lin, Y., Shi, Y., . . . Lu, Z. (2022). Identification of multi-omic biomarkers from fecal DNA for improved detection of colorectal cancer and precancerous lesions. Unpublished manuscript
Disclaimer
- The content is provided for educational and informational purposes only and does not constitute providing medical advice or professional services. It should not be used for diagnosing or treating a health problem or disease; those seeking personal medical advice should consult a licensed physician.
- The product described or shown is only available in certain countries.