Table of Contents
Introduction
Typical Reactions
Possible Drug-Drug Interactions
Approaches as Per Bio-Analytical Chemistry
Example Including Chiral Metabolism
Enantio-Selective Metabolism at EAG Laboratories
References
Introduction
“In order to understand the actions of drugs, it is an absolute necessity to have knowledge of the transformations they undergo in the body. We must not judge drugs according to the form and amount administered, but rather according to the form and amount which actually is eliciting the action.”
Rudolph Buchheim (1820–79), one of the pioneers of experimental pharmacology wrote the above statement in 1859. It underscores the importance of pharmacokinetics, pharmacodynamics and especially the role of metabolite identification and profiling.
Not many aspects of metabolite profiling and drug metabolism are more crucial than exploring chiral-selective (also referred to as stereo-selective) metabolism. For many decades of the century, study of chirality in drug metabolism was limited to academic projects and to herbal products due to the limitations of separations chemistry. However, chirality has been gaining more importance in drug development, as many new molecules entering the market in the 21st century are single enantiomers, unlike the achiral drugs that subjugated the second half of the 20th century. Chiral-selective metabolism has significant impact on drug safety, pharmacokinetics, pharmacology, and bio-analytical chemistry; and also on regulatory, patents, business development, and other vital considerations.
Typical Reactions
While direct chiral-selective effects on the physicochemical aspects are minimal, there always exist impact on metabolism. This is applicable to the drug substances, products, and also to multiple enzyme systems (such as P450). For example:
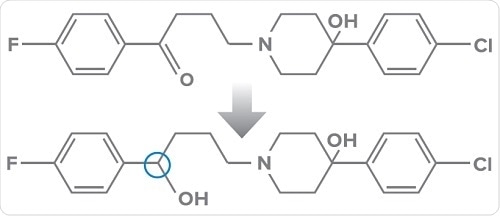
The conversion of haloperidol to a chiral product via achiral metabolism (carbonyl reduction) (S-hydroxy-haloperidol is preferred in humans) (above);
- Chiral to chiral conversion of (S)-warfarin to (S)-7- or (S)-6-hydroxywarfarin;
- Chiral to achiral conversions, such as transformation of 1,4-dihydropyridine calcium antagonists to the respective pyridine analogs;
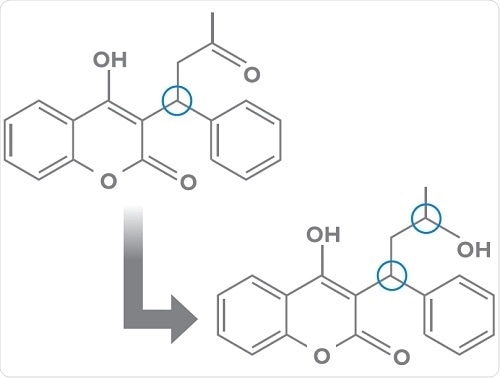
- Chiral to diastereomer conversions such as transformation of warfarin to two pairs of diastereomeric alcohols via keto-reduction (below);
- Enzymatic and non-enzymatic chiral inversions for example, transformations occurring with 2-arylpropionic acid NSAIDS.
Possible Drug-Drug Interactions
Dispensing achiral drugs is polypharmacy, by definition. When another drug is added, the effects on pharmacology, drug interactions, or other safety aspects can be significant when inhibition or induction of one drug can influence the PK behavior another drug, and highlights the importance of exploring chirality in metabolite profiling techniques.
Such as the interaction of warfarin and cimetidine [reduces the clearance of (R)-warfarin] or warfarin and sulfinpyrazone [reduces the clearance of (S)-warfarin]. Similarly, achiral mixtures can also have effects on pharmacodynamics and/or safety:
- The activity of each enantiomer could be different, and each of them can be developed based on their merits;
- One of the enantiomers may be efficacious, while the other one may be biologically inert;
- Two enantiomers could have conflicting effects on the same target;
- Both enantiomers may show pharmacological activity, but one of them may have adverse safety profile.
Approaches as Per Bio-Analytical Chemistry
As stated before, an advancement in column chemistry technique for achieving necessary separations has been a driving force in the increased importance of chiral-driven PK and pharmacodynamics. It has been said that the “development of complex PK models and plasma–concentration–effect relationships based on ‘total’ drug concentrations following administration of a racemate are of limited value and potentially useless.”
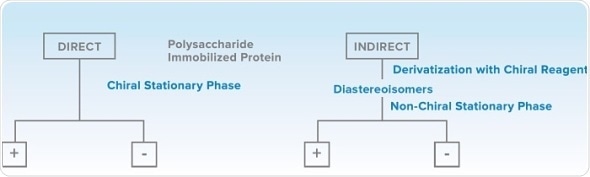
Bio-Analytical Chemistry Approaches
Any quantitative analytical chemistry method should aim for reproducibility and accuracy, with quick sample preparation, and use of suitable mobile phases or pH. However, special attention is needed for development and validation of methods for chiral separations. Direct separation of enantiomers using a chiral stationary phase (such as immobilized protein or polysaccharide) is preferred. Indirect separation method using derivatization of enantiomers with a chiral reagent employing conventional column to produce diastereoisomers can be an alternative to the direct approach.
Example Including Chiral Metabolism
Current advancement towards single enantiomers as suitable drugs, should result in reduction of drug interactions which are usually associated with achiral mixtures. This, however, does not nullify the challenges concerning chiral-to-chiral, achiral-to-chiral and/or chiral-to-diastereomer conversions.
A hypothetical study (based on anonymized actual data) of chiral metabolism and metabolite profiling, is explained below.
Assume a small molecule drug which shows efficacy in a mouse model and also demonstrates an acceptable PK and safety profiles during preclinical studies. Early metabolite profiling studies in mouse have shown that the most important pathway was keto-reduction forming the corresponding alcohol (similar to the haloperidol reaction stated above), but with a chiral center now:
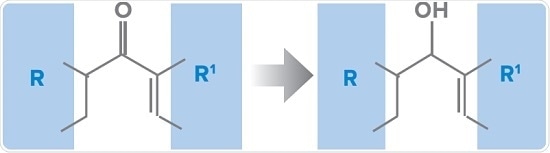
According to these findings, possible chiral metabolism to the chiral alcohol should be looked for, with the following vital questions to be considered:
- Is there chiral (to the S- or R-form) metabolism to the corresponding alcohol?
- What are the results of in vitro metabolism in other preclinical species and humans?
- Can a bio-analytical method be developed for the enantiomers with quick sample preparation?
- Can the method be optimized and validated?
- Do the in vivo outcomes match with the in vitro study results?
- Which enzyme is involved in the metabolism?
- Is there any impact on business development/regulatory//intellectual property?
Preferred metabolism of a keto-reduced metabolite to the S-form has been demonstrated in in vitro experiments (liver S9) in multiple species (>80% in mouse; >90% in dog, rat, and human). However, the same was not seen in monkeys: this could have a great impact on process of selection of species for toxicity studies. PK studies in rat, mouse, and dog were consistent with the results of in vitro studies: that is, the preferred enantiomer was the S-form, and in conformance with the ratios noted in the in vitro studies. Also, the keto-reduced metabolite had several-fold higher plasma exposure compared to the parent entity in other species (which creates questions for possibility of a “disproportionate metabolite”). Finally, the S-form of the metabolite was also noted to have good efficacy on the target.
From analytical point of view, initial techniques employed an immobilized protein column which offered very good separation of chiral forms of keto-reduced metabolite, but demanded long (>60 minutes) runtime, and yielded inferior peak shapes and sensitivity. The techniques were not robust enough to put up with repeated injections. Subsequent attempts resulted in successful utilization of different chiral column which allowed for shorter run time and enhanced sensitivity along with cost-effectiveness.
The above hypothetical case is a good example of the requirement to consider chiral-selective metabolism and its potential implications on pharmacodynamics, PK, toxicology, and bio-analytical chemistry; and also on business development, regulatory, intellectual property, and other important aspects.
Enantio-Selective Metabolism at EAG Laboratories
EAG laboratories has more than three decades of experience in conduct of ADME (absorption, distribution, metabolism, and excretion) studies in preclinical species to support drug development.
We design and monitor the in-life stages at partner sites with AAALAC-accredited facilities which are vetted and qualified by EAG. Dose preparation, mass balance calculations, preparation of samples, metabolite profiling, extractions, and identification of unknown metabolites are carried out by highly experienced scientists at EAG. Our scientists can also aid related services including quantitative whole body autoradiography.
Specific capabilities include:
- Preparation of samples and development of extraction methods
- Chromatographic method development
- Experts in significant publications in drug metabolism
- Radiolabeled and non-labeled ADME studies – preclinical or clinical trial support
- Radiochromatographic profiling
- Identification with LC-MS/MS (also high resolution mass spectrometry)
- Proposal of metabolic pathways
- Stable-labeled, radio-labeled, or non-labeled synthesis of drugs and their metabolites
References
- A. Conti and M. H. Bickel, Drug Metab. Rev., 1977, 6, 1–50.
- V. Campo, L. Bernardes and I. Carvalho, Curr. Drug Metab., 2009, 10, 188–205.
- D . Brocks, Biopharm. Drug Dispos., 2006, 27, 387–406.
- Chirality in Drug Design and Development, ed. I. Reddy and R. Mehvar, CRC Press, New York, 2004.
- A . Hutt, Metab. Drug Interact., 2007, 22, 79–112.
- Adapted from Campo, et al. (ref. 2, above)
Hypothetical Case Study: The author has had the privilege of working on several interesting examples of stereo-selective metabolism in his career. The hypothetical case study presented in this white paper is based on one of those examples; additional data on the example is reported here:
- J. Schmidt, A. Nouraldeen, L. Moran, L. Li and A. Wilson, Enantio-selective and Species-Dependent Carbonyl Reductase Metabolism of LX6171, 12th Annual Conference on Drug Metabolism and Applied Pharmacokinetics, 2009.
- L. Li, W. Heydorn, J. Kramer, A. Nouraldeen, J. Schmidt, J. Jiang, L. Moran and A. Wilson, Metabolism Mediated CYP2B Induction by LX6171 (3′-chlorobiphenyl-4-yl)-1-(pyrimidine-2-yl) piperidin-4-yl methanone in the Rat, International Society for the Study of Xenobiotics (ISSX) Annual Meeting, 2009.
Other recent case studies are reported here:
- J. Schmidt, “Metabolite Profiling,” in A.G.E. Wilson (ed.), New Horizons in Predictive Drug Metabolism and Pharmacokinetics, RSC Publishing, Cambridge, UK, 2015

This information has been sourced, reviewed and adapted from materials provided by EAG Laboratories.
For more information on this source, please visit EAG Laboratories.