Jul 12 2018
Prostate-specific antigen or gamma-seminoprotein is produced by the epithelial cells of the prostate gland and is commonly used as an indicator of prostate function. The current test measures PSA protein in blood but accurate diagnosis of prostate cancer using this test alone remains challenging, due to the high sensitivity and low specificity of the PSA test- which may present as raised in other disorders such as infection.
However, the expression of PSA mRNA and other validated biomarkers such as PCA3 in urine and blood are far more suitable for diagnosis but they also have drawbacks and limitations in terms of ease of sample handling. This is because they require the urine sample to be immediately frozen, otherwise nucleic acids may be broken down before they can be detected.
ARCIS Technology
ARCIS-Biotechnology offer a solution to this problem, with their novel technology that prepares, stabilizes and processes urine samples for RNA-based screening. This involves a process in which the sample is mixed with the ARCIS reagent to rapidly stabilize and protect the mRNA biomarkers within the urine,
The sample preparation is typically achieved in under three minutes in a simple two-step process and allows subsequent detection of genetic markers using a Reverse Transcription Quantitative Polymerase Chain Reaction (RTqPCR) assay.
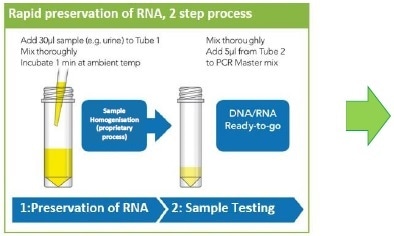
Figure 1. Rapid sample processing. Urine samples were collected in preservation reagent immediately prior to storage for stability assessment.
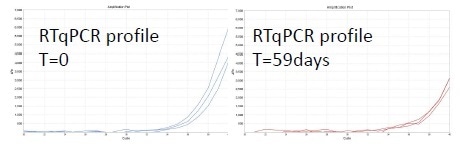
Figure 2. qRT-PCR results.
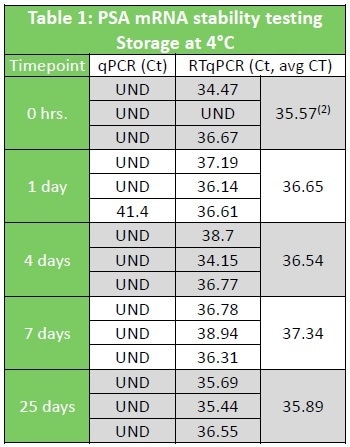
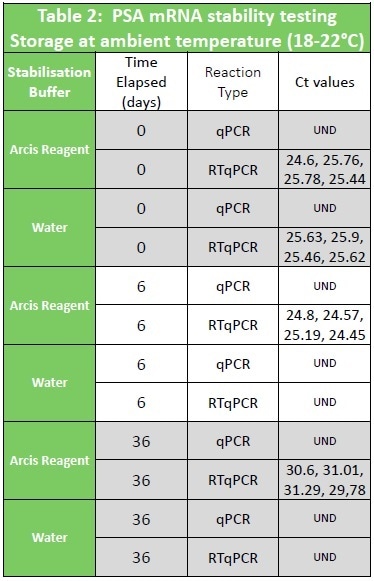
Table 1 and Table 2 show data from Urine samples stored in Arcis preservation reagent. De-protection of the sample for molecular analysis is a single pipette step into Wash Buffer.
Aiding Diagnostic Tests for Prostate Cancer
The major advantage of the ARCIS RNA and DNA extraction process is that, in contrast to current approaches, genomic contents within the urine sample have been shown to remain stable at room temperature without needing preservation below freezing. This is due to ARICS reagent 1, which modifies the conformation of nucleic acids and prevent their degradation by nucleases.
Studies testing the ARCIS technology have confirmed the specificity of this approach in producing an accurate measure of the PSA coding gene expression, which in turn can be used to infer information about the function of the prostate gland.
Additional genetic markers can also be extracted and identified using this novel method, such as PCA3 and Beta-actin. Such markers may be highly useful in aiding a multiplexed detection of synergistic oncogenic markers for prostate cancer diagnosis.
Conclusion
Identifying the presence of PSA mRNA in urine can be used as a diagnostic test for prostate cancer. ARCIS have developed technology that stabilizes RNA within urine samples over an extended time-course, paving the way for a simpler and more convenient clinical diagnostic test for cancer.
 About Arcis Biotechnology
About Arcis Biotechnology
Arcis Biotechnology is a research and development led company with expertise in the development and commercialization of a wide range of fast and convenient sample preparation technologies.
Based at Sci-tech Daresbury, where we have fully equipped laboratory facilities, the Group has already developed a number of patented compounds and technologies that have been enthusiastically received in the commercial marketplace via our licensing partners.
Arcis Biotechnology is focused on providing fast and convenient nucleic acid sample preparation solutions. Obtaining genetic material from biological samples is a critical step in molecular biology processes which is often time consuming and inefficient.
In less than 3 minutes with no prior sample preparation the Arcis Sample Prep range of products allow users to quickly and conveniently go from biological material to downstream nucleic acid investigation using PCR, qPCR, RT-qPCR or sequencing without the need for traditional isolation or purification.
Arcis DNA Isolation Video
The process requires no laboratory equipment and requires as little as 30 µl of blood as a starting material. Arcis products can be used on many different samples containing DNA or RNA including whole blood, plasma, urine, buccal swabs, hair follicles and microbiological samples including bacteria (E. coli, S. aureus, P. aeruginosa, K. pneumonia), viruses (HBV/ HCV) and parasites (plasmodium).
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.