Jul 12 2018
Next Generation DNA Sequencing (NGS) technologies are highly beneficial in aiding clinical diagnosis due to the simultaneous detection of multiple pathological targets. However, the processes involved in this; including DNA extraction, amplification, sequencing and subsequent data analysis, are complex and time-consuming.
Therefore, in order to provide rapid identification of pathogens, the workflow of NGS technologies must be accelerated. This is especially important for diagnosis of infectious diseases such as sepsis, where the speed of diagnosis and administration of appropriate treatments are major predictors of survival.
Rapid Sample Preparation
One way to increase the speed of clinical NGS workflow is with rapid sample preparation. This can be achieved using a novel two-step system produced by ARCIS-Biotechnology, which allows preparation, stabilization and extraction of samples for DNA-based screening.
Research comparing this sample prep method to a leading column-based DNA extraction kit observed similar performance between both extraction techniques, and produced genomic sequences that were successfully compatible for subsequent detection of genetic markers using Polymerase Chain Reaction (PCR).
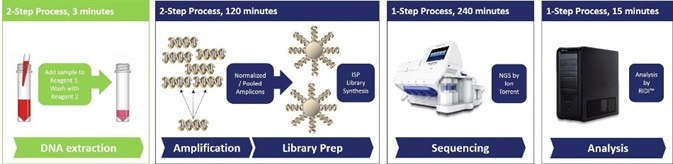
It was found, however, that sample preparation time was reduced from 60 minutes with a standard extraction kit to three minutes using the ARCIS system. This confirms that the novel two-step extraction system is able to rapidly process DNA samples without compromising on quality.
Streamlined Data Analysis
The speed of NGS techniques can also be improved at the stage of data analysis. RIDI, for instance, is a rapid data analysis system developed by Fry Laboratories that rapidly performs all data processing steps including DNA sequencing filtering, trimming and editing.
This is important because many processes within NGS, such as sequence amplification using PCR, cannot be achieved at a faster rate. In contrast, speedy analysis of genetic data obtained from molecular techniques can easily reduce the overall time of workflow using advanced and streamlined analysis software.
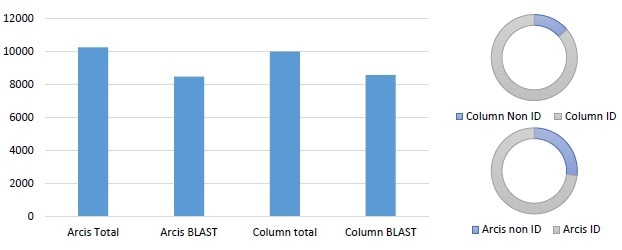
Figure 1. Similar performance observed between column extraction and Arcis extraction (E. coli spiked into blood). The graph shows the total number of sequences generated, and the number with valid BLAST returns. The pie charts indicate how many sequences match the target species within 95% or greater. Processing time for the column based kit was 60 minutes, processing time for the Arcis kit was 3 min.
Table 1. PCR Protocol Optimisation.
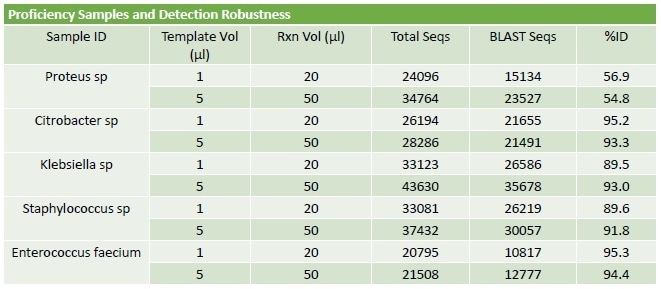
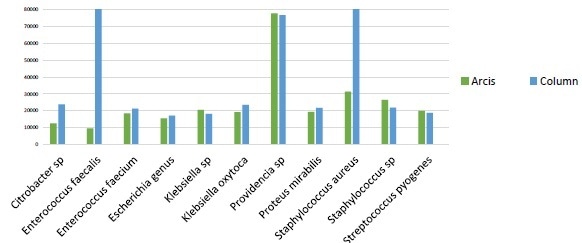
Figure 2. Similar performance observed between column extraction and Arcis extraction on a panel of proficiency testing samples, representing a range of Gram positive and Gram negative bacteria. Two outliers to the data were Enterococcus faecalisand Staphylococcus aureus, which gave considerably more sequences on the column-based extraction system. The RIDI data analysis software rapidly performed all data processing steps including sequencing filtering, trimming and editing.
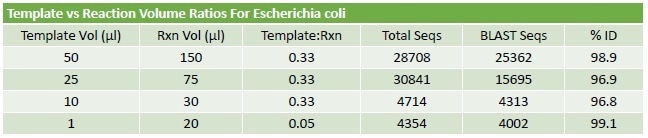
Table 2. PCR Protocol Optimisation. An assessment of input template volume and total reaction volume suggests that the protocol is robust with good performance across a range of input values. Once a set of trimmed reads is obtained from the RIDI software, the sequences are identified using a unique reductive BLAST strategy. The % ID results, generated on E.coli spiked blood, were greater than 96% regardless of protocol used.
Conclusion
Combining rapid sample prep using novel technology by ARCIS with RIDI data analysis software can save considerable amounts of time in an NGS workflow, and therefore can aid the molecular diagnosis of infectious diseases in clinical labs.
 About Arcis Biotechnology
About Arcis Biotechnology
Arcis Biotechnology is a research and development led company with expertise in the development and commercialization of a wide range of fast and convenient sample preparation technologies.
Based at Sci-tech Daresbury, where we have fully equipped laboratory facilities, the Group has already developed a number of patented compounds and technologies that have been enthusiastically received in the commercial marketplace via our licensing partners.
Arcis Biotechnology is focused on providing fast and convenient nucleic acid sample preparation solutions. Obtaining genetic material from biological samples is a critical step in molecular biology processes which is often time consuming and inefficient.
In less than 3 minutes with no prior sample preparation the Arcis Sample Prep range of products allow users to quickly and conveniently go from biological material to downstream nucleic acid investigation using PCR, qPCR, RT-qPCR or sequencing without the need for traditional isolation or purification.
Arcis DNA Isolation Video
The process requires no laboratory equipment and requires as little as 30 µl of blood as a starting material. Arcis products can be used on many different samples containing DNA or RNA including whole blood, plasma, urine, buccal swabs, hair follicles and microbiological samples including bacteria (E. coli, S. aureus, P. aeruginosa, K. pneumonia), viruses (HBV/ HCV) and parasites (plasmodium).
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.