Data security and the maintenance of appropriate electronic records is essential for GxP labs. Hound from Unchained Labs can help meet this need thanks to its 21 CFR Part 11 compliant software package, providing data authenticity, user accountability, and full sample tracking.
Hound ensures that any lab can meet compliance with 21 CFR Part 11 regulations. It also includes features such as system and data integrity checks, controlled user access, electronic signatures, and experiment-specific audit trails.
Hound utilizes a combination of microscopy, dual Raman spectroscopy and laser-induced breakdown spectroscopy (LIBS) to count and identify specific particles (Figure 1). Counting provides information on size and morphological properties of individual particles. Dual Raman at 785 nm and 532 nm focuses on the identification of protein, organic, and inorganic particles with LIBS used to identify elemental and metal particles.
Samples can be prepared individually, with filter rounds isolating particles for chemical ID, adhesive rounds isolating particles for elemental ID, and wet rounds ensuring that particles remain in suspension while counting and identification take place.

Figure 1. Hound counts and identifies the composition of visible and sub-visible particles with both automated and manual modes. Hound uses Raman (532 nm and 785 nm) and LIBS to identify the composition of particles, helping users track down the particle source.
21 CFR part 11 main screen
The main 21 CFR Part 11 screen may be accessed at any time in the Hound Client software. This is accomplished by clicking on the appropriate tab from the drop-down menu on the home page (Figure 2).
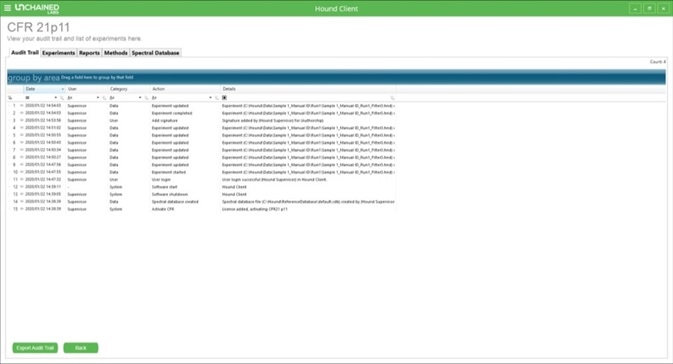
Figure 2. Main 21 CFR Part 11 screen in Hound Client.
The following key features are available as standard:
- Controlled, secure user access: The administrator can set password requirements, define user access levels, and create or disable user accounts as appropriate.
- Audit trails: Every event or action undertaken by users - including changes to the software such as user settings, reports, or experiments - is recorded in the database.
- Digital signatures: A signature in the form of login credentials is required before an experiment begins.
- Integrity check: This feature triggers a check of all experiments, databases, method files, and reports for integrity, informing the user if any have been corrupted or changed without proper authorization.
The 21 CFR Part 11 main screen offers an overview of the audit trail. Users can sort for specific parameters by applying a series of filters (Table 1), and the audit trail can be exported as a PDF (either with or without these filters applied) by clicking on the “Export Audit Trail” button.
Table 1. A list of actions in the audit trail by which a user can filter or sort.
| System |
User |
Data |
Spectral database |
| Activate CFR |
Add signature |
Experiment analyzed |
Spectral database file closed |
| Database integrity check |
Add signature (canceled) |
Experiment completed |
Spectral database file created |
| Software shutdown |
Add signature (failed) |
Experiment opened |
Spectral database file opened |
| Software start |
New user-created |
Experiment recount reverted |
Spectral database file updated |
| Surface correction updated |
User login |
Experiment recounted |
Spectral database closed |
| |
User logout |
Experiment spectrum processed |
Spectral database created |
| |
|
Experiment spectrum reverted |
Spectral database default updated |
| |
|
Experiment started |
Spectral database opened |
| |
|
Experiment updated |
Spectral database removed |
| |
|
Method created |
Spectral database updated |
| |
|
Report created |
|
| |
|
Report updated |
|
Experiment-specific information and audit trails
Opening the Hound Analysis software then clicking on the “CFR21p11” tab will display an overview of all completed experiments (Figure 3). This list will also show the last user to modify each experiment.
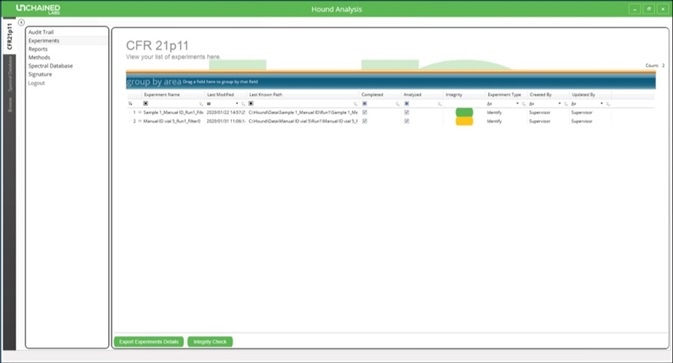
Figure 3. Experiment list in the Hound Analysis software showing an example of one experiment that failed the integrity check and is colored yellow.
An integrity check may be performed on the list of experiments, which will prompt any experiments that have been modified without the proper authorization to appear in yellow. When any user or administrator performs an experiment, an authorization signature is required to confirm authorship (Figure 4).
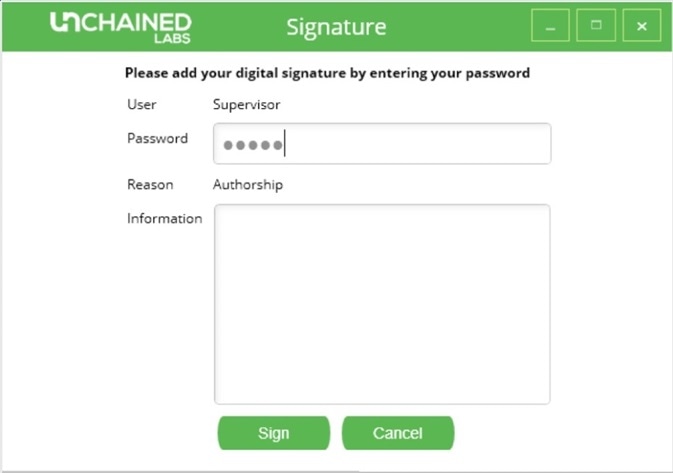
Figure 4. Digital signature request screen in Hound Analysis software.
The administrator and user are also able to sign all experiments for responsibility, review, and approval. Each time an experiment file is opened, the aforementioned integrity check is rerun to determine whether or not that particular experiment has been compromised. Any report details are automatically generated, including the experiment’s file name, the sample name, and any analysis settings. Users cannot modify any text or data in this report to ensure report integrity.
The Hound CFR database includes experiment identification information which is specific to the data integrity of all experiment files. Each computer will have its own individual license, so files created on one 21 CFR Part 11-active computer cannot be transferred to another without compromising their integrity. An Unchained Labs employee can transfer the CFR database a new computer if this is required, however, and this transfer can be completed while maintaining the integrity of the data.
Conclusion
In order to comply with GxP laboratory requirements, Hound Client and Analysis software offer features in line with 21 CFR Part 11 regulations. These features include electronic storage of data as HDF files, from which the user can create reports at any time.
The audit trail ensures that archives of experiments and reports are kept while providing an overview of all actions performed by a user. Integrity checks may be performed on databases, experiments, reports, and method files to identify any altered or corrupted files.
Additional features include comprehensive audit trail capabilities, an advanced user management system, and signature requirements on all electronic records.
About Unchained Labs
Unchained Labs are all about helping biologics researchers break free from tools that just don’t cut it. Unleashing problem-tackling products that make a huge difference in the real science they do every day.
That’s their mantra, their promise and they own it. They live by an unconventional strategy for a start-up: they're buying commercial businesses and developed technologies, adding their magic touch to turn them into breakthrough products, investing massively in customer-facing teams and then selling those products like gangbusters.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.