A significant parameter to explore the compressibility and flowability of pharmaceutical powders, tapped density, is extremely useful for promoting the approach of QbD and GMPs. Therefore, standardization of apparatus and procedure is vital to get meaningful repeatable results.
In this process, a BeDensi T3 Pro was used with three workstations, performing standardized tapped density tests of three excipients. It is important to note that this highly efficient and economic tester is designed to be fully compliant with USP and EP standards.
The main components of pharmaceutical powders are pharmaceutical ingredients (API) and excipients, which are subsequently formed and presented to the patient as solid dosages in forms like powder, tablets and capsules.
Powders with certain characteristics are deemed to have good compressibility, which means that they are suitable to be made into tablets through the process of compression in a tableting press.
The key to the success of many pharmaceutical operations, including tableting and capsule filling, is the flow properties of the powders. Such powders easily promote the API’s homogeneity and excipient bulk with the appropriate flow properties.
When it comes to promoting the approach of Quality by Design (QbD) and good manufacturing practices (GMPs) for pharmaceutical products and processes, knowing these properties is useful.
One of the necessary parameters needed to calculate the compressibility index (CI) or Hausner ratio (HR), which relates to the flow characteristics of the pharmaceutical powders, is tapped density (volume).
The tapped density (X) of a powder is the name given to the ratio of the mass of powder (M) to the volume (VF) occupied by powder after it has undergone the process of tapping for a defined period. The tapped densities of samples are calculated using the equation below:
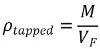
Tapped density test is a popular method – thanks to its simplicity and convenience – when it comes to understanding powder flow properties. There has been harmonization and standardization of the test recommended by the European Pharmacopoeia (EP) and the United States Pharmacopoeia (USP), and thus of apparatus and procedure in order to attain reliable and essential results.
This article outlines a standardized tapped density test of excipients, according to USP and EP standards.
Standardized test
Apparatus
The BeDensi T Pro series is fully compliant with EP and USP standards and can help users to easily and accurately perform standardized tests of tapped density.1, 2 In addition, user-defined measurements are also supported.
Table 1. Table 1 displays the compliance of the BeDensi T Pro series. Source: Bettersize Instruments Ltd.
| USP & EP Standards |
Methods Ⅰ |
Methods Ⅰ/Ⅱ |
BeDensi T Pro Series |
| Cylinder |
Volume (mL) |
250 |
25 |
100 |
250 |
| Mass (g) |
220 ± 44 |
55 ± 10 |
140 ± 10 |
210 ± 10 |
| Height (mm) |
≤335 |
155 ± 10 |
245 ± 10 |
237 ± 10 |
| Tap |
Height (mm) |
14 ± 2 |
3 ± 0.2 |
3 ± 0.2 or 14 ± 2 |
| Speed (taps/min) |
300 ± 15 |
250 ± 15 |
100-300 (adjustable) |
Note: A 100-ml graduated cylinder can be used for less than 100 g sample.

Image Credit: Bettersize Instruments Ltd.
Test procedure
The BeDensi T3 Pro was used with three workstations to obtain the tapped volume of three common excipients (namely microcrystalline cellulose MCC, lactose and mannitol) by the USP Method Ⅰ (procedure displayed in the following schematic).
75 g MCC, 120 g lactose and 120 g mannitol were used in order to conform to the volume requirement (≥60% of 250 ml cylinder volume) of USP or EP.
Until the point at which the difference between succeeding test volumes (Δ V) is less than or exactly 2 mL, the latter is the tapped volume. The BeDensi T Pro will calculate the tapped density automatically after inputting the tapped volume and the mass value. After that, the CI and HR can also be calculated according to the following formula:
CI = 100 (V0 - VF)/VF
HR = V0/VF
Where V0 is the unsettled volume, and VF is the tapped volume.

Figure 1. The test procedure. Image Credit: Bettersize Instruments Ltd.
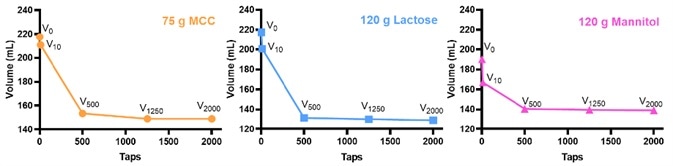
Figure 2. The volume change during tapping. Image Credit: Bettersize Instruments Ltd.
Table 2. Flowability confirmation. Source: Bettersize Instruments Ltd. Source: Bettersize Instruments Ltd.
| Excipient |
Bulk
Density (g/mL) |
Tapped
Density (g/mL) |
CI |
HR |
Flowability |
| MCC |
0.34 |
0.50 |
32 |
1.47 |
Very poor |
| Lactose |
0.55 |
0.93 |
41 |
1.69 |
Very, Very poor |
| Mannitol |
0.63 |
0.86 |
27 |
1.37 |
Poor |
Results
The BeDensi T3 Pro obtained volumes of the three samples in question under different taps. The volume decreased with an increasing number of taps, as evidenced by the diagram. The volume change only decreased slightly when the number of taps reached 500. The V2000 is the tapped volume for MCC while V1250 is the tapped volume for lactose and mannitol.
CI, HR, the bulk density and the tapped density were confirmed (as evidenced by Table 2). The flow behavior of these excipients was determined on the basis of the relationship between the CI/HR and the flowability.3 When the MCC and mannitol were compared, it was clear that the lactose had the poorest flowability in this case.
Conclusion
By using the BeDensi T3 Pro, standardized tests of tapped density were able to be performed and facilitate the study of tapped density of the three pharmaceutical powders.
Thanks to the equipment’s accuracy and reliability, the tapped density and tapped volume of these samples were obtained easily and scientifically. Therefore, it is necessary to use a reliable tapped density tester like the BeDensi T Pro series to attain significant and valuable results for evaluating the flowability.
References
- USP <616>, Bulk Density and Tapped Density of Powders
- Eur. Ph. 2.9.34. Bulk Density and Tapped Density of Powders
- USP <1174> Powder Flow
About Bettersize Instruments Ltd.

With over 25 years experience developing and manufacturing particle characterization instruments, Bettersize has introduced breakthrough technology in the field of particle size & shape measurement.
By achieving high quality and superior performance, our instruments provide precise analysis results of particle size, particle shape, and powder characteristics, helping scientists and engineers to understand material properties, facilitate research and improve production efficiency.
Bettersize product line for particle size and shape analysis includes instruments of all needs and budgets, from basic to advanced research models. These instruments are widely applied in Pharmaceuticals, Battery materials, Mining and minerals, Metals, Chemicals and Surface coatings, measuring materials with size ranges from nanometer to millimeter.
Focused on technology innovation, instruments manufacturing, application support and after-sales services, Bettersize provides expertise and professional solutions and assures customers the highest confidence in our products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.