Iron dextran is a liquid complex that consists of ferric hydroxide and dextran, which is utilized in the treatment of iron deficiency anemia via intramuscular injection. In a clinical setting, it is injected into patients for whom oral iron supplements are not suited, or for those who require immediate and sufficient iron supplements.

Image Credit: Bettersize Instruments Ltd.
The size and size distribution of iron dextran is closely associated with the manufacturing process of the preparation, and consequently, influences the bioavailability, efficacy, and immune response of the final product.
Moreover, product stability can be assessed by monitoring the size and size distribution of the preparation. This article presents a comparison study on the size distributions of one commercially available and one R&D stage iron dextran.
Instrumentation
The BeNano 90 nanoparticle size analyzer (Bettersize Instruments Ltd.) fitted with a solid-state laser source of a wavelength of 671 nm and a power of 50 mW was used.
An avalanche photodiode (APD) detector was employed to pick up scattered light signals at 90 degrees. Single-mode fibers were introduced for signal transmission for the purpose of increasing the signal-to-noise ratio.
Experiment
During this study, commercially available and R&D stage iron dextran was measured. The stock solutions of each sample were dark-brown suspensions, signifying high absorbance qualities.
Dilution of the stock solutions was carried out using deionized water by 100 times, respectively, and the measurements were conducted at 25 °C with the integrated temperature control unit in the BeNano 90.
Each sample was subjected to at least three measurements to acquire the standard deviations of sizes and assess the result repeatability.
Results and discussion
By evaluating the original scattered light signals, the correlation functions of samples are determined, as displayed in Figure 1 and Figure 2.
As shown, both correlation functions demonstrate high signal-to-noise ratios and exceptional repeatability. However, their decay rates indicate slight deviations from one another, as presented in Figure 3.
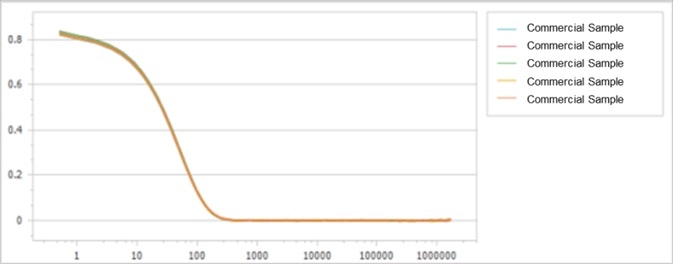
Figure 1. Correlation function of commercially available sample. Image Credit: Bettersize Instruments Ltd.
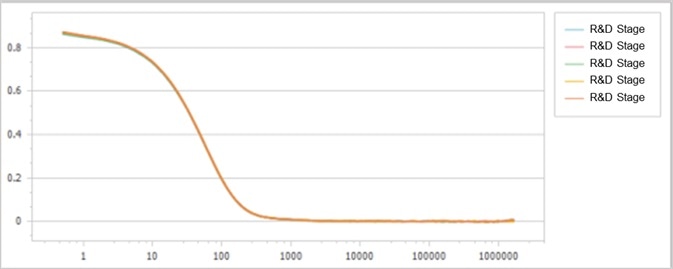
Figure 2. Correlation function of the R&D stage sample. Image Credit: Bettersize Instruments Ltd.
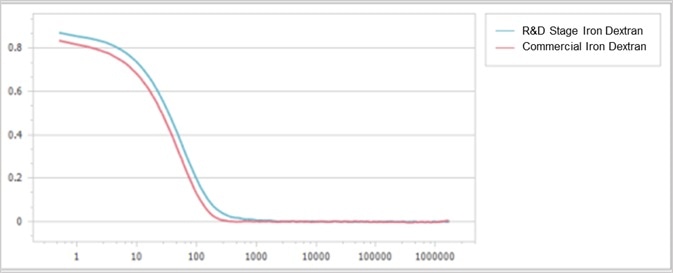
Figure 3. Correlation function comparison between two samples. Image Credit: Bettersize Instruments Ltd.
The commercially available sample’s correlation function deteriorated more rapidly than that of the R&D stage sample. This, in turn, indicates that the size of the R&D stage iron dextran was larger than the commercially available sample and its particles experienced slower diffusion.
Table 1 presents how the R&D stage iron dextran exhibited a larger Z-average size and larger polydispersity index (PD.I), in contrast to the commercially available one.
Table 1. Size and size distributions of iron dextrans. Source: Bettersize Instruments Ltd.
| Iron Dextran Sample |
Z-average Size (nm) |
PD.I |
| Commercially Available |
15.78 ± 0.27 |
0.089 |
| R&D stage |
2.25 ± 1.26 |
0.230 |
Size distributions of two iron dextran preparations are presented in Figure 4 and Figure 5, establishing the narrow distribution of sizes in the commercially available sample, compared with the two peaks from the R&D stage sample.
Such an additional peak at around 200 nm indicates that there were aggregates present in the R&D stage sample.
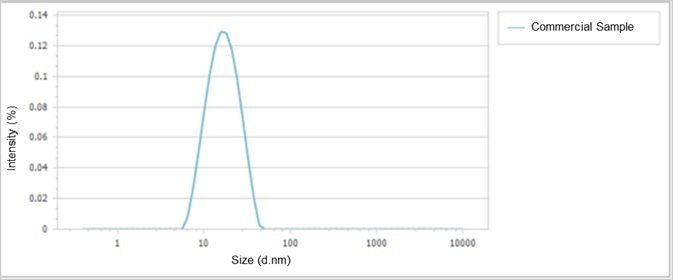
Figure 4. Size distribution of the commercially available iron dextran. Image Credit: Bettersize Instruments Ltd.
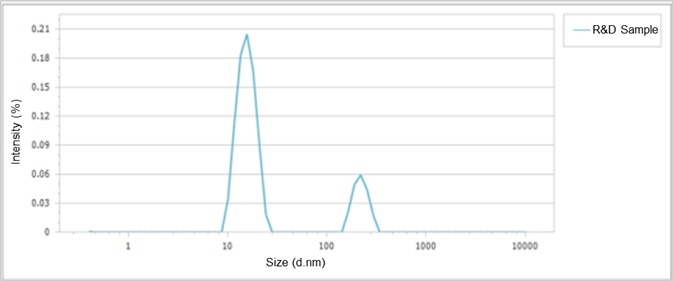
Figure 5. Size distribution of the R&D stage iron dextran. Image Credit: Bettersize Instruments Ltd.
Conclusions
This study demonstrates how the BeNano 90 was utilized for the characterization of two iron dextran injections: a commercially available one and an R&D stage one. Size differences were successfully distinguished, and the presence of aggregates in the R&D sample was ascertained.
With respect to the injection preparations, careful attention needs to be paid to the formation of aggregates, as they can severely influence the drug stability, efficacy, and immune response.
In conclusion, the application of the BeNano 90 with its outstanding sensitivity for aggregates or large particles will be greatly useful and handy as a research tool for injection preparation.
About Bettersize Instruments Ltd.

With over 25 years experience developing and manufacturing particle characterization instruments, Bettersize has introduced breakthrough technology in the field of particle size & shape measurement.
By achieving high quality and superior performance, our instruments provide precise analysis results of particle size, particle shape, and powder characteristics, helping scientists and engineers to understand material properties, facilitate research and improve production efficiency.
Bettersize product line for particle size and shape analysis includes instruments of all needs and budgets, from basic to advanced research models. These instruments are widely applied in Pharmaceuticals, Battery materials, Mining and minerals, Metals, Chemicals and Surface coatings, measuring materials with size ranges from nanometer to millimeter.
Focused on technology innovation, instruments manufacturing, application support and after-sales services, Bettersize provides expertise and professional solutions and assures customers the highest confidence in our products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.