Sponsored Content by PromoCellReviewed by Alex SmithJan 24 2023
Innovative mouse research is essential for improving cancer therapies. Multiple biological data collection strategies are required for translating scientific discoveries from mice to men. This article will illustrate how.
In vivo, in vitro, and ex vivo cell culture techniques play an essential role in disclosing vital information that will assist the oncology industry’s progress, from attempting to comprehend the processes of cancer stem cells to instituting personalized cancer therapies. Robust cell culture methods are especially required for the research of cancer biology.
When researching cancer therapies, patient-derived xenograft (PDX) mouse and rat models are largely relied on to offer crucial information on the efficacy and safety of drug candidates.1
However, the PDX model is expensive, time-consuming, and laborious for high-throughput drug screenings, and it is limited to in vivo application. Despite the initial promise, most drug prospects fail in the clinical trial stage. Ex vivo cell culture approaches will ideally aid to boost the relevancy of data and the efficacy of laboratory operations compared to in vivo studies.
This article investigates the benefits and constraints of the PDX mouse model, as well as strategies for overcoming existing problems by ex vivo, expanding PDX-derived cells in 2D and 3D cell culture models for various in vitro screening and analysis assays.
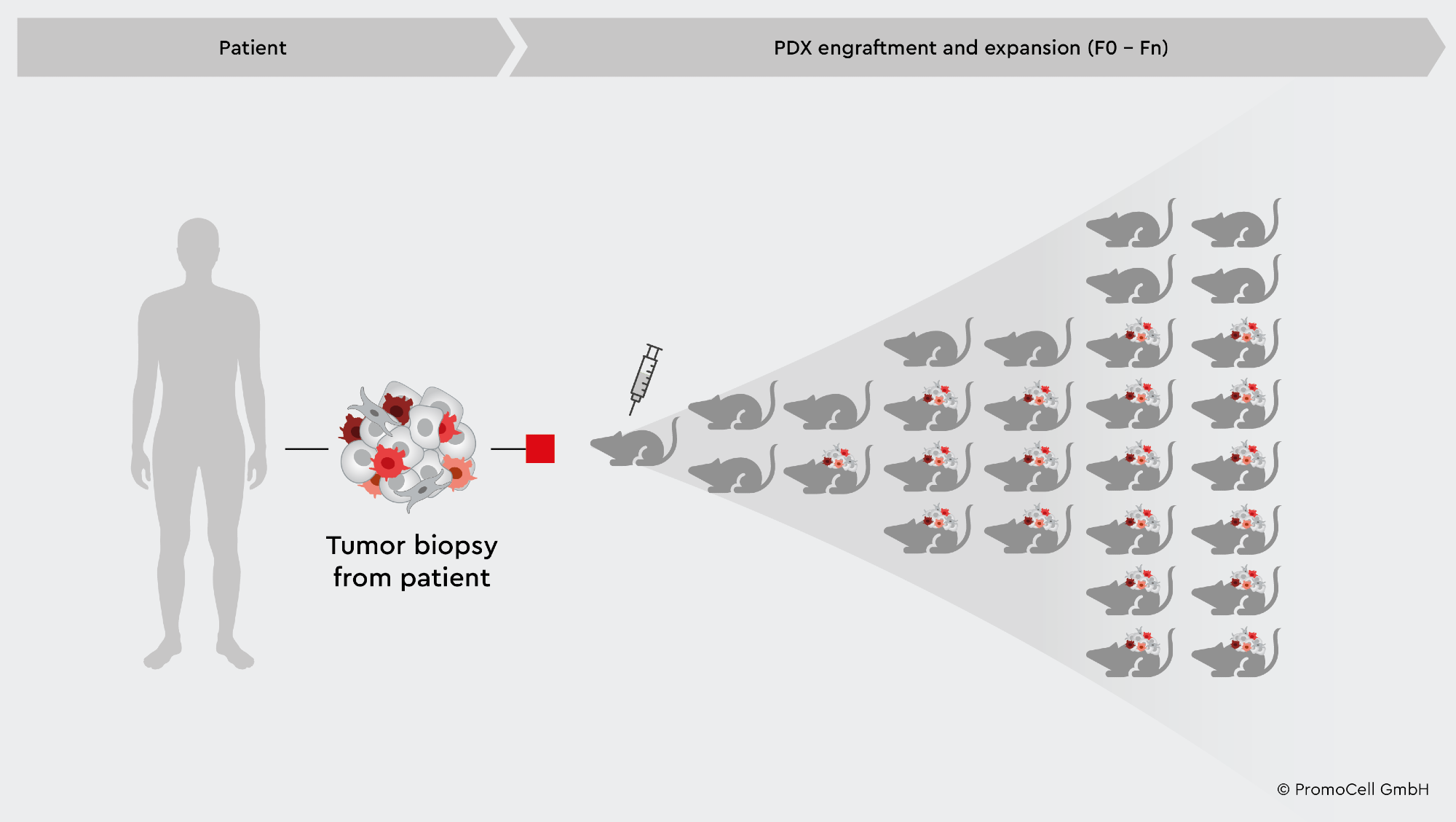
Image credit: PromoCell GmbH
Advantages of the PDX mouse model
PDX is produced by implanting patient tumor samples into immunocompromised animal models, often mice or rats, which are then repeatedly transplanted to promote cancer development and progression.2
PDX models retain original patient tumor characteristics, such as cell structure, gene expression, metastatic potential, and genetic stability, which may then be examined in an environment that closely replicates how cancer operates in humans.1,2
This results in an excellent live target population of the study. PDXs can be employed as a successful platform to predict clinical responses to future cancer therapies since the models provide a valuable reference of research for the disease with translatable findings to human systems.2
PDX models are also useful since they can be used to design individualized cancer treatments. PDX models, for instance, can be developed from cancer patients undergoing clinical trials and receiving the same treatment as the patient to detect potential biomarkers or treatment responses to increase accuracy in medicine.2
Because of the PDX model’s strengths, it is a popular choice for cancer biology research. However, as with many laboratory techniques, there are places for development, and supplementing or broadening investigations to include several types of cell culture methodologies results in more robust and reliable findings.
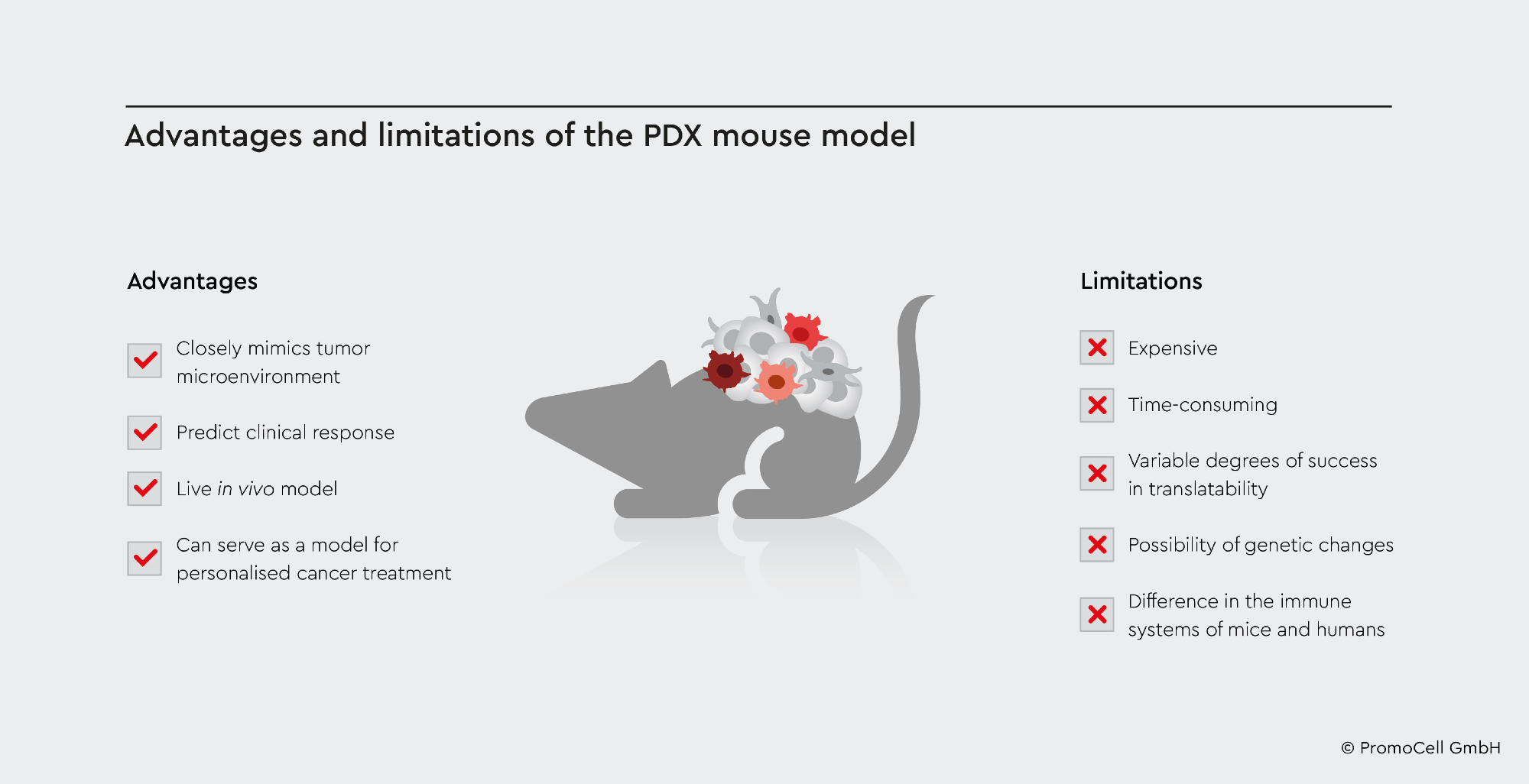
Image credit: PromoCell GmbH
Limitations of the PDX mouse model
Using PDX murine models can be expensive financially and in terms of resources. It is also thought to be time-consuming, with varying degrees of effectiveness in patient translation.3
Tumors, for instance, could fail to develop and metastasize and lose some of the original features of the transplanted tumor samples, undermining the study’s translatability.2
Tumors are highly heterogeneous and depend solely on samples to engraft and transplant into mice. As a result, they may not accurately depict human tumor morphology, resulting in a lack of vital information on how that cancer may behave and interact in a biological system, impacting the translatability value of PDX models.2 When cancer is transplanted into a new host, genetic alterations might occur, impacting the genetic features of the original tumor.2
As the mice used in PDX have a compromised immune system, they cannot accurately represent what would occur in the case of the human immune system.2 Moreover, the tumor microenvironment in PDX models is poorly known and develops over time, which could result in differing biological responses between mice and humans when researching cancer drugs.3
These shortcomings must be addressed, given the inconsistent success in developing viable PDX models and the fact that drug candidates in clinical trials are examined entirely on PDX data.
Ex vivo studies utilizing PDX material for cell culture preserve the original tumor biology, make sure that findings are translatable, and offer a more cost-effective and less time-consuming method of extracting physiologically relevant data, allowing for more intensive research into drug molecules in clinical trials.1
Innovating solutions to PDX with ex vivo cell culture
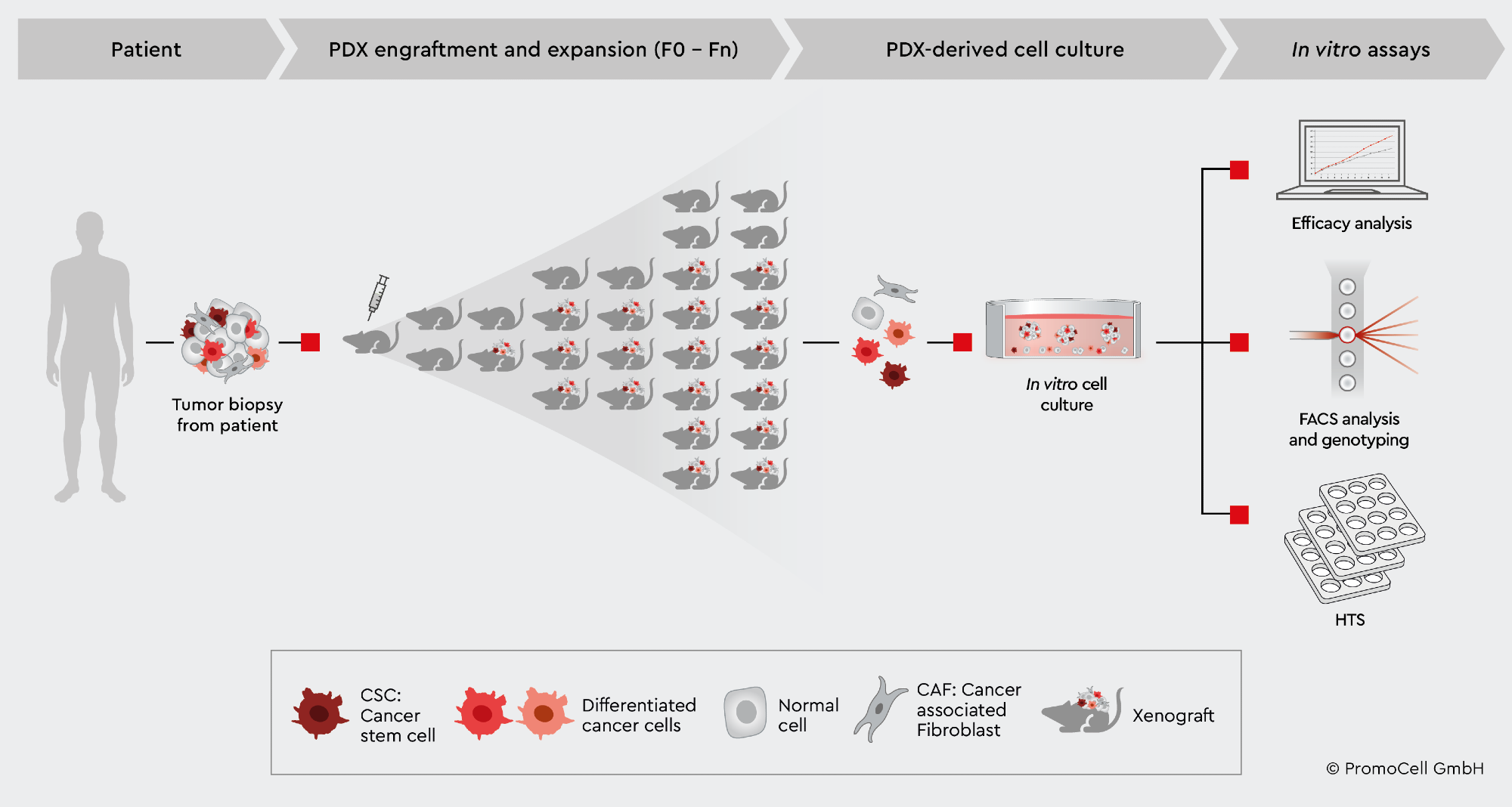
Image credit: PromoCell GmbH
Ex vivo cell culture investigations are particularly important for improving the comparability of specific results from PDX models and providing answers to concerns that may emerge during animal model trials.1 Ex vivo cell culture, for example, provides a platform to pinpoint areas of improvement for the molecule after detecting a therapeutic failure in a PDX model before conducting a second round of PDX studies.1
The experts of PromoCell have created a cancer media portfolio that includes several functional media that enable users to create ex vivo 2D and 3D tumor models from PDX mouse-derived tumor models. The portfolio includes media solutions for producing primary cell lines from PDX models for testing and screening assays, creating cell master stocks for future studies, and developing 3D tumor models such as spheroids and organoids to address more complex tumor biology concerns.4,5
It is vital to have a reliable method of growing PDX-derived cancer cells ex vivo to make maximum use of PDX mouse models. When separating cancer cells from biopsies, mouse cells are a regular problem with traditional medium. Cancer cells are rapidly overrun by stroma cells, and mouse cells, particularly in PDX-derived cell cultures, influence data interpretation.
This problem can be handled by extracting primary human cancer cells from PDX and then growing them for additional tests. Because PromoCell’s Primary Cancer Culture System excludes mouse cells, only human cells proliferate in cell culture following isolation.6
References
- Making best use of your PDX mouse. (2022, September 22). PromoCell. https://promocell.com/research-areas/applications-for-our-cancer-media-toolbox/making-best-use-of-your-pdx-mouse/
- Pompili, L., Porru, M., Caruso, C., Biroccio, A., & Leonetti, C. (2016). Patient-derived xenografts: a relevant preclinical model for drug development. Journal of Experimental & Clinical Cancer Research, 35(1). https://doi.org/10.1186/s13046-016-0462-4
- Primary Cancer Cells: A Culture System Opens the Door to Individualized Therapy. (2017, December 11). PromoCell. https://promocell.com/cells-in-action/primary-cancer-cells-a-medium-opens-the-door-to-individualized-therapy/
- Cancer stem cells in tumor biology. (2022, November 7). PromoCell. https://promocell.com/blog/cancer-stem-cells-in-tumor-biology/
- A Home for Cancer Stem Cells: 3D Tumorspheres. (2018, April 17). PromoCell. https://promocell.com/in-the-lab/a-home-for-cancer-stem-cells-3d-tumorspheres/
- Primary Cancer Culture System. (2022, August 10). PromoCell. https://promocell.com/product/primary-cancer-culture-system/
About PromoCell
Advances in science and medicine are powered by human endeavor. PromoCell empowers your biomedical research. We are your partners for primary cells and cell culture media, as well as for cell biology research. Our portfolio comprises more than 7,000 products that follow the strictest ethical and quality standards.
With more than 28 years of experience in human primary cell culture, we deliver research tools for scientists worldwide. Because sharing knowledge is key to scientific discovery, we also offer professional training at our PromoCell Academy. Our team of experts is by your side in your quest for new therapies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.