Sponsored Content by MaxCyte, Inc.Reviewed by Maria OsipovaMar 31 2023
Natural killer (NK) cells, the immune system's first responders, are vital for the quick detection and elimination of cancer. Their capacity to kill cancer cells without prior activation makes them an ideal candidate for immunotherapy.
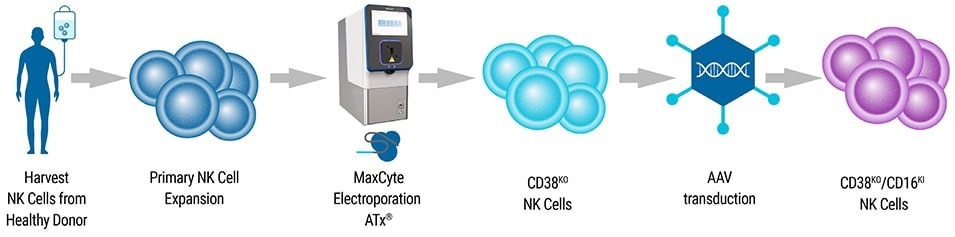
Image Credit: MaxCyte, Inc.
NK cells trigger an anti-tumor immune response called antibody-dependant cellular cytotoxicity (ADCC) when activating receptors, such as CD16, attach to monoclonal antibodies (mAbs) on target cells. Combining adaptive NK cell infusions with mAbs is a promising potential cancer treatment.
Daratumumab (DARA) is a monoclonal antibody (mAb) that adheres to CD38 and has shown clinical success in treating multiple myeloma, making it an attractive candidate for enhancing NK cell infusion. Yet, as circulating NK cells display high amounts of CD38, DARA can induce ADCC-dependent NK fratricide, reducing the efficiency of this treatment combination.1
Combining DARA with the adoptive transfer of CD38 knockout (CD38KO) NK cells can potentially increase ADCC and reduce DARA-induced fratricide, boosting NK cell persistence. To further enhance their potency, NK cells can be engineered to express a high-affinity CD16 (CD16-158V) receptor, which has been demonstrated to increase ADCC, resulting in enhanced clinical results 2.
This article demonstrated how MaxCyte® enabled multiplexed genetic editing in NK cells. Dual-edited NK cells enhanced DARA treatment and improved anti-tumor responses in vitro and in vivo.
The advantages provided by MaxCyte electroporation, include high efficiency, minimal toxicity, and clinical scalability, which enabled the rapid development of an effective cancer treatment.
Aim
This innovative research from the Child's laboratory used complementary genetic engineering methods to produce NK cells with enhanced ADCC potency and resistance to fratricide in the presence of DARA.
The findings reveal that MaxCyte electroporation had a minimal impact on cell survival and function and provides a safe platform for the development of cell-based cancer therapeutics.
Methods
- Harvest: NK cells were isolated from healthy donor peripheral blood buffy coat.
- Expansion: Ex vivo expansion of isolated NK cells was conducted for one week. NK cells were cocultured with irradiated Epstein–Barr virus-transformed lymphoblastoid feeder cells and IL-2.
- Electroporation: The MaxCyte ATx® and NK-04 electroporation protcols were employed to deliver pre-complexed Cas9 and CD38-targeting gRNA to expanded NK cells. Knockin (KI) of CD16-158V was performed using two distinct approaches: electroporation and AAV transduction.
- Transient CD16 Expression: The ATx® was employed to transfer CD16-158V mRNA to CD38KO NK cells, seven days after initial electroporation, resulting in transient dual-edited NK cells.
- Stable CD16 KI: CD38KO NK cells were transfected with rAAV6 with an HDR template encoding the CD16-158V gene with an N-terminal FLAG-tag 30 minutes after the first electroporation. Transduction led to stable integration of the CD16-158V transgene at the CD38 locus.
- Characterization: Engineered cells were isolated and characterized using PCR and flow cytometry. The functionality of isolated NK cells was determined by an in vitro killing assay and in murine models of multiple myeloma.
Results
Highly efficient gene knockout
MaxCyte® allowed for effective CD38 deletion in 92% of expanded NK cells (Fig 1A). Electroporation was gentle on cells since their viability remained high days after transfection (Fig 1B).
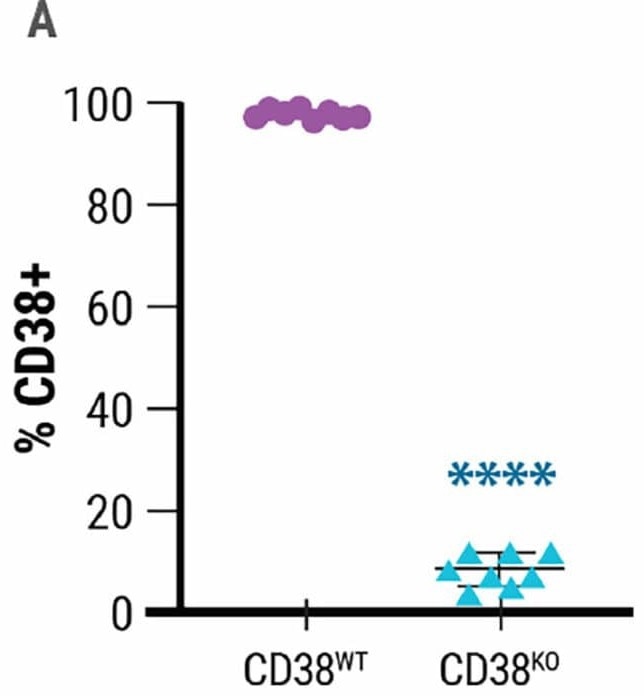
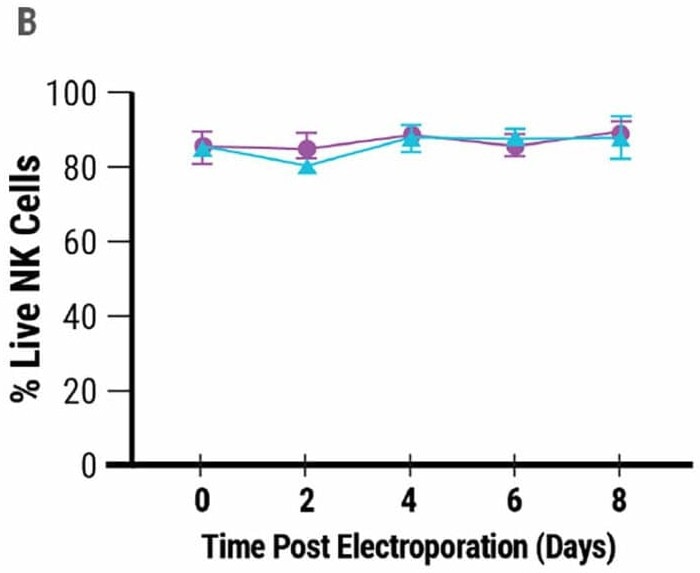
Figure 1. High-efficiency gene editing A) Relative expression of CD38 in wild-type (CD38WT) and CD38KO NK cells. B) Annexin staining and flow cytometry were used to determine NK cell viability 8 days post electroporation. Image Credit: MaxCyte, Inc. adapted from Joseph Clara et al. J Immunother Cancer. (2022) under Creative Commons License Atrribution 4.0 International (CC BY 4.0)
Characterization of CD38KO NK cells
Neither the proliferative nor functional features of NK cells were altered by genetic engineering. Ex vivo growth rates of non-edited and CD38KO NK cells were comparable (Fig. 2A), as was the expression of an array of NK surface receptors after electroporation (Fig 2B).
Degranulation was measured by the production of lysosomal-associated membrane protein-1 (CD107a) on NK cells in the presence of their target cells and was comparable in CD38KO and CD38WT cells. (Fig 2 C).
In the presence of target cells, the generation of intracellular IFNy (Fig. 2D) and TNF (Fig. 2E) indicated that the NK cellular cytotoxicity was similar between unmodified and engineered NK cells.
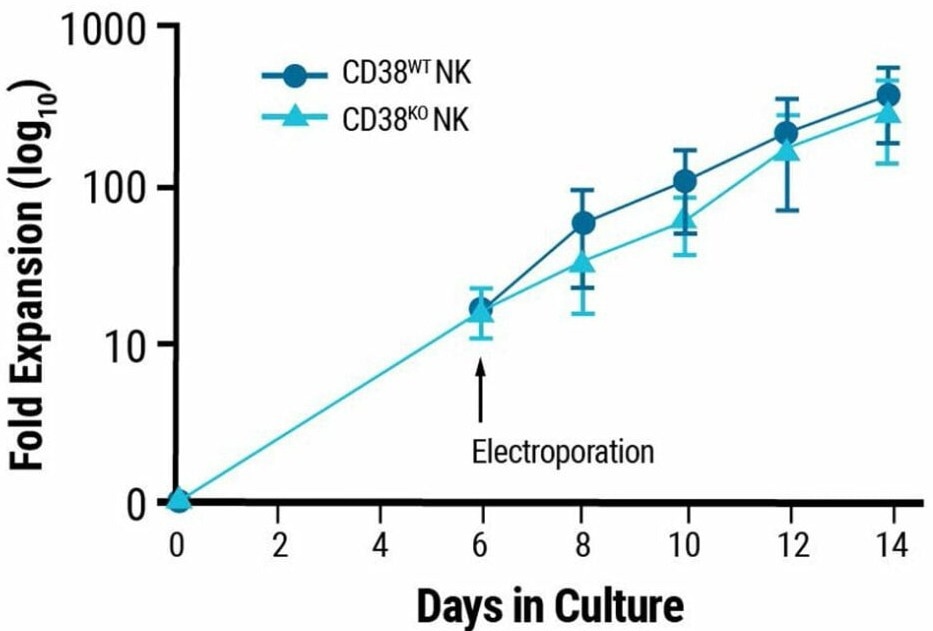
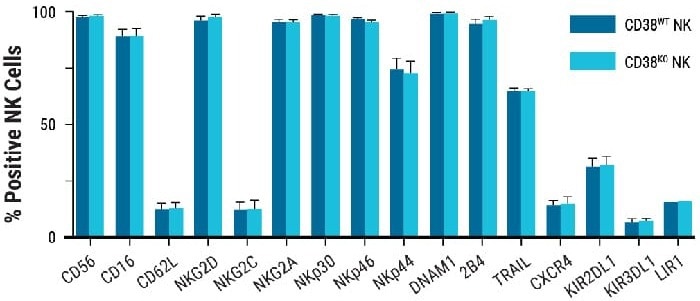
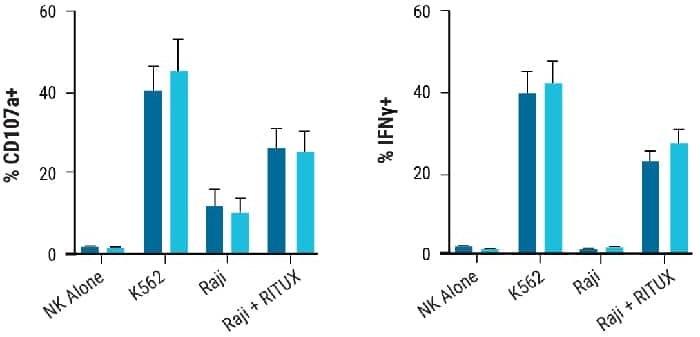
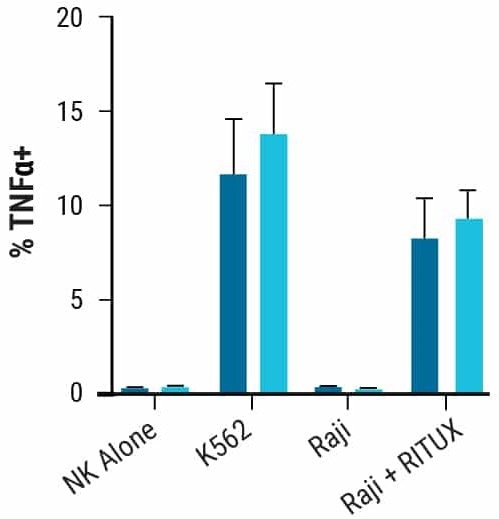
Figure 2. NK cell characterization post electroporation Proliferative and functional characteristics of NK cells remain unchanged post electroporation. A) CD38KO and CD38WT NK cell expansion 14 days post electroporation. B-E) Marker expression in CD38KO and CD38WT NK cells following coculture with target cells (K562 or Raji cells) was determined using flow cytometry. Raji cells treated with rituximab (RITUX) were used as a positive control. B) Surface marker expression in CD38KO and CD38WT NK cells. C) Degranulation was measured via CD107a expression. D-E) NK cytotoxicity was determined through intracellular INF-ү and TNF-α expression. Image Credit: MaxCyte, Inc.adapted from Joseph Clara et al. J Immunother Cancer. (2022) under Creative Commons License Atrribution 4.0 International (CC BY 4.0)
Arming CD38KO NK cells
To examine if the introduction of CD16-158V could increase the efficacy of CD38KO NK cells, CD16-158V mRNA was electroporated into CD38KO NK cells.
When cocultured with CD38+ NCI-H929 myeloma cells treated with DARA, CD38KO NK cells transfected with CD16-158V mRNA exhibited higher cytotoxicity (Fig 3). MaxCyte electroporation was used to demonstrate that the expression of CD16-158V might increase the efficacy of CD38KO NK cells.
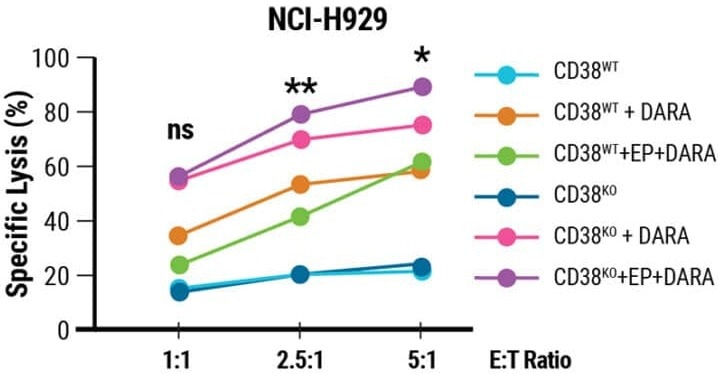
Figure 3. Enhanced functionality of CD38KO NK with CD16-158V expression Cell lysis of DARA-treated NCI-H929 cells in the presence of CD38KO NK cells with (CD38KO +EP) or without CD16-158V expression one day post electroporation at various effector-to-target (E:T) ratios. Image Credit: MaxCyte, Inc.adapted from Joseph Clara et al. J Immunother Cancer. (2022) under Creative Commons License Atrribution 4.0 International (CC BY 4.0)
Multiplexed engineering
Stable multiplexed engineering was achieved through the combination of electroporation and AAV transduction. CRISPR-Cas9 RNP electroporation was employed to silence CD38 while a recombinant AAV provided an HDR template expressing CD16-158V.
Production of double-edited CD38KO/CD16KI NK cells was determined by surface FLAG expression; 58% of CD38KO cells expressed CD16-158V (Fig 4A).
Double-edited CD38KO/CD16KI NK cells in combination with DARA induced enhanced cell lysis in multiple myeloma target cells (Fig 4B) and demonstrated the highest reduction in tumor burden in MM1.S xenograft mice, indicating superior anti-tumor activity (Fig 4C).
MaxCyte's electroporation technology can be combined with complementary genetic engineering tools, such as AAVs to develop the next generation NK cell threapeutics.
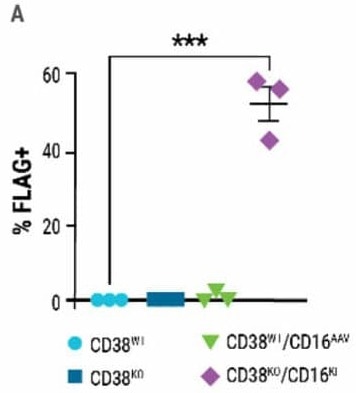
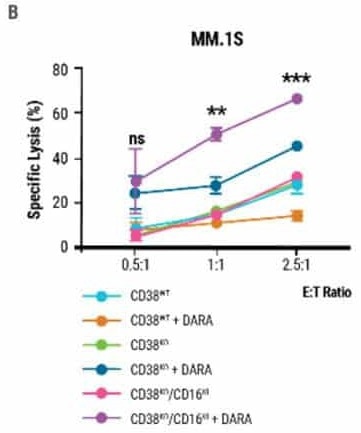
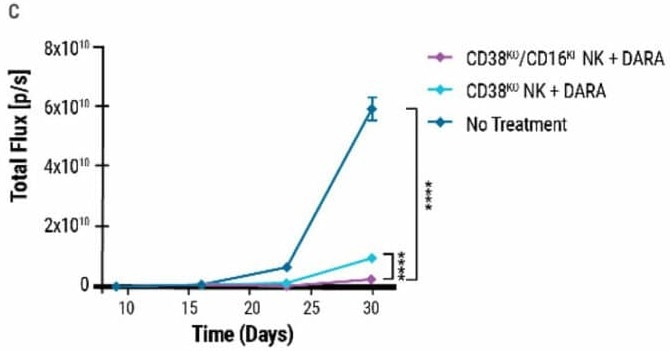
Figure 4. Double-edited NK cells have superior anti-tumor activity A) Percentage of FLAG-positive NK cells measured 7 days post CRISPR-Cas9 RNP electroporation and AAV transduction. B) Comparison of double-edited (CD38KO/CD16KI), single-edited (CD38KO) and unedited (CD38WT) NK cells’ ability to enhance DARA-mediated lysis in MM.1S target cells. C) Double-edited NK cells demonstrated higher anti-myeloma activity in xenograft mice. The MM.1S cells used to form the tumor were lentivirally transduced to express firefly luciferase. Bioluminescent quantification (photons per second or total flux) of MM.1S myeloma tumor growth was measured over 30 days. Image Credit: MaxCyte, Inc. adapted from Joseph Clara et al. J Immunother Cancer. (2022) under Creative Commons License Atrribution 4.0 International (CC BY 4.0)
Conclusion and future applications
Coupling monoclonal antibody therapy with highly effective adoptive NK cell transfer is a promising cancer treatment stratgey. The combination of MaxCyte's electroporation technology with AAV transduction enabled the development of an easily adaptable workflow for multiplexed genetic engineering in NK cells.
Using MaxCyte® electroporation, the remarkable efficiency of CD38 knockout removed the need for additional enrichment steps for engineered cells. Moreover, the decreased in CD38 expression was stable over time and provided resistance to DARA-induced NK fratricide.
Electroporation did not compromise the functional or proliferative capabilities of engineered NK cells. Moreover, MaxCyte electroporation was used to arm CD38KO cells with the high-affinity CD16-158V receptor, boosting their cytotoxicity in the presence of DARA.
Electroporation and AAV transduction together led to the effective generation of double-edited NK cells. CD38KO/CD16KI NK cells increased the antitumor efficacy of DARA in vitro and in vivo .
MaxCyte enabled in the development of a streamlined methodology for multiplexed engineering of natural killer (NK) cells, which, when paired with DARA, provides an interesting combinatorial immunotherapeutic approach well suited for clinical applications.
References
- Wang Y, Zhang Y, Hughes T, et al. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin Cancer Res. 2018;24(16):4006-4017. doi: 10.1158/1078-0432.CCR-17-3117.
- Clara JA, Levy ER, Reger R, Barisic S, Chen L, Cherkasova E, Chakraborty M, Allan DSJ, Childs R. High-affinity CD16 integration into a CRISPR/Cas9-edited CD38 locus augments CD38-directed anti-tumor activity of primary human natural killer cells. J Immunother Cancer. 2022 Feb;10(2):e003804. doi: 10.1136/jitc-2021-003804.
About MaxCyte, Inc.
MaxCyte is a leading commercial cell-engineering company focused on providing enabling platform technologies to advance innovative cell-based research as well as next-generation cell therapeutic discovery, development and commercialization. Over the past 20 years, we have developed and commercialized our proprietary Flow Electroporation® platform, which facilitates complex engineering of a wide variety of cells.
Our ExPERT™ platform, which is based on our Flow Electroporation technology, has been designed to support the rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes: four instruments, the ATx™, STx™, GTx™, and VLx™; a portfolio of proprietary related processing assemblies or disposables; and software protocols, all supported by a robust worldwide intellectual property portfolio.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.