The amino acid side chains of proteins undergo various post-translational modifications (PTMs) which significantly impact protein function and mediate complex cellular events.
Measuring the dynamics, diversity, and functional consequences of PTM states of proteins across the proteome is fundamental to understanding the function of proteins in health and disease.
However, the discovery and detection of PTMs and routine measurement of complex PTM states is extremely challenging, and the diversity of proteoforms in the human proteome continues to be mostly unmapped.1
Novel and more sensitive approaches to PTM detection will considerably help the discovery of biomarkers and drugs and the development of precise and personalized approaches to medicine.
Arginine side chain modifications are of significant biomedical interest. Methylation and citrullination of arginine residues in various human proteins have been crucial in disease states, including autoimmune disease, cardiovascular disease, and cancer.2-6
This article presents the utilization of Quantum-Si’s next-generation protein sequencing technology for detecting arginine methylation and citrullination with single-molecule resolution and sensitivity.7
The next-generation protein sequencing technology provided by Quantum-Si delivers a sensitive platform for detecting and discovering PTMs. This addresses the crucial demand for accessible techniques for examining the role of PTMs in human health and disease.
Arginine is vital in the structure and function of proteins because of the guanidinium group's unique properties that form the terminus of its side chain.
This group is positively charged and can form extended hydrogen bond networks and cation-π interactions with other amino acids and nucleic acids. As a result of this, arginine frequently facilitates important interactions between proteins and DNA or between protein-binding partners.
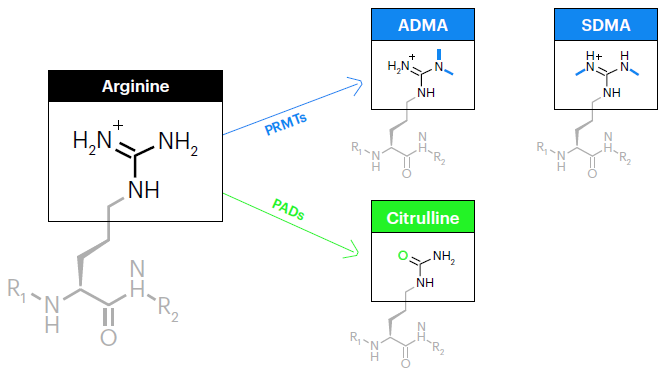
Figure 1. Post-translational modifications of arginine. The arginine side chain consists of a positively-charged guanidinium group at the terminus of a flexible aliphatic chain. In arginine dimethylation, PRMTs transfer two methyl groups - either asymmetrically to the same nitrogen atom (ADMA) or symmetrically onto opposite nitrogen atoms (SDMA). These modifications not only increase size and hydrophobicity but also modulate hydrogen bonding. In arginine citrullination, PADs carry out the hydrolysis of arginine’s positively-charged guanidinium group, resulting in a neutral ureido group a transformation referred to as deimination. This transformation results in a negligible mass increase of 0.9840 Da, though the loss of positive charge can dramatically alter protein conformation and function. Image Credit: Quantum-SI
The two most common arginine PTMs, citrullination and dimethylation, change the arginine side chain and its properties, as displayed in Figure 1. This may lead to critical downstream effects on cellular processes.
Dimethylation preserves the positive charge of arginine but increases both its hydrophobicity and size, and blocks the formation of hydrogen bonds.
Citrullination removes the positive charge of arginine, leading to a neutral side chain. This significantly affects protein conformation and function.
Dimethylation and citrullination of arginine are conducted by enzymes and may be part of the normal regulation of cellular processes or involved in disease states.
The dimethylation of arginine is catalyzed by protein arginine methyltransferases (PRMTs). These transfer two methyl groups either symmetrically onto opposite nitrogen atoms, leading to symmetric dimethylarginine (SDMA), or asymmetrically onto the same nitrogen atom, leading to asymmetric dimethylarginine (ADMA).
The citrullination of arginine is catalyzed by protein arginine deiminases (PADs). These conduct the hydrolysis of the positively-charged guanidinium group of arginine, leading to a neutral ureido group.
Arginine PTMs are now recognized as essential targets within biomedical research. Methylated arginine residues and their respective PRMTs have been demonstrated to be involved in diseases, including cancers and cardiovascular disease.2,3
Research has shown that arginine citrullination is critically involved in immune system function, myelination, skin keratinization, and gene expression regulation.4-6 In some cases, eliminating arginine’s positive charge can result in proteins activating the immune system, contributing to autoimmune diseases.5
Technological challenges in the detection of arginine PTMS
Investigating these arginine PTMs has been challenging because their detection and differentiation are complex using current proteomic methods.8 Mass spectrometry is the most popular tool for detecting protein PTMs.
However, mass spectrometry cannot easily differentiate between SDMA and ADMA because they are constitutional isomers with identical masses.9 Similarly, deimination of arginine to citrulline leads to a negligible mass increase of 0.9840 Da.
This mass difference may be easily confused with a 13C isotope or mistaken to be the deamidation of nearby glutamine or asparagine residues.10 Mass spectrometry techniques for arginine PTM detection also demand highly specialized knowledge, training, and advanced analysis techniques.
Enzyme-linked immunosorbent assay (ELISA) is another popular technique for PTM detection. This technique utilizes antibodies that are specifically produced for the detection of a modified protein of interest.
Arginine PTMs are estimated to be widespread in human cells, but commercially available antibodies against arginine PTMs are restricted to specific sites on a few highly studied proteins.11
The requirement to generate new antibodies, as well as complex workflows, high costs, limited antibody reproducibility, and other challenges linked with ELISA assay development, will likely hinder the discovery and further research of novel arginine PTM sites.12
The continued development of novel methods is required to assist the direct detection of arginine PTMs in proteins.
Single-molecule protein sequencing provides an alternative approach to the detection of SDMA, ADMA, and citrulline that is not based on mass-to-charge ratio or antibody specificity, but instead on the kinetic signature of binding between recognizers and N-terminal amino acids (NAAs).
Quantum-Si’s next-generation protein sequencing platform provides deeper insights into these PTMs with single-molecule resolution, addressing present gaps in technology, and enabling the direct detection of arginine PTMs.

 Click here to download the whole version of the paper
Click here to download the whole version of the paper
References and further reading
- Aebersold, R. et al. (2018) How many human proteoforms are there? Nature Chemical Biology 14, 206–214
- Couto e Silva, A., Wu, C. Y.-C., Citadin, C. T., Clemons, G. A., Possoit, H. L. E., Grames, M.S., Lien, C.-F., Minagar, A., Lee, R. H.-C., Frankel, A., and Lin, H. W. (2019) Protein arginine methyltransferases in cardiovascular and neuronal function. Molecular Neurobiology 57, 1716–1732
- Chen, L., Liu, S., and Tao, Y. (2020) Regulating tumor suppressor genes: Post-translational modifications. Signal Transduction and Targeted Therapy 5, 1–25
- James, E. A., Pietropaolo, M., and Mamula, M. J. (2018) Immune recognition of ß-cells: Neoepitopes as key players in the loss of Tolerance. Diabetes 67, 1035–1042
- Nguyen, H., and James, E. A. (2016) Immune recognition of citrullinated epitopes. Immunology 149, 131–138
- Wegner, N., Lundberg, K., Kinloch, A., Fisher, B., Malmström, V., Feldmann, M., and Venables, P. J. (2010) Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunological Reviews 233, 34–54
- Reed, B. D. et al. (2022) Real-time dynamic single-molecule protein sequencing on an integrated semiconductor device. bioRxiv
- Witze, E. S., Old, W. M., Resing, K. A., and Ahn, N. G. (2007) Mapping protein post-translational modifications with mass spectrometry. Nature Methods 4, 798–806
- Afjehi-Sadat, L., and Garcia, B. A. (2013) Comprehending dynamic protein methylation with mass spectrometry. Current Opinion in Chemical Biology 17, 12–19
- Vitorino, R., Guedes, S., Vitorino, C., Ferreira, R., Amado, F., and Van Eyk, J. E. (2020) Elucidating citrullination by mass spectrometry and its role in disease pathogenesis. Journal of Proteome Research 20, 38–48
- Larsen, S. C., Sylvestersen, K. B., Mund, A., Lyon, D., Mullari, M., Madsen, M. V., Daniel, J. A., Jensen, L. J., and Nielsen, M. L. (2016) Proteome-wide analysis of arginine monomethylation reveals widespread occurrence in human cells. Science Signaling 9
- Egelhofer, T. A. et al. (2010) An assessment of histone-modification antibody quality. Nature Structural & Molecular Biology 18, 91–93
About Quantum-SI
Inspired by Ion Torrent’s success at shrinking next-generation sequencing technology into a benchtop instrument, Jonathan Rothberg founded Quantum-Si™ to bring the same semiconductor technology to protein sequencing with the launch of the Platinum® Next-Generation Protein Sequencer™.
That was in Guilford, CT, back in 2013. Fast forward to today and we now have over 1,000 patents issued and applications pending, plus a groundbreaking single-molecule protein sequencing technology platform, the Platinum.
Along the way, we solved critical challenges around sensitive and unambiguous amino acid detection, blending biology, chemistry, and semiconductor technology to help biologists see what other approaches cannot deliver. We also set the stage for a revolution in how scientists understand biology and build new treatments for disease by making single molecule protein sequencing accessible to every lab everywhere.
We are now entering a new phase of our development as a company. Starting with an initial public offering in June 2021 (QSI on the NASDAQ) and continuing with a new product development and operations facility in San Diego, CA, in 2022, we have entered a period of rapid growth. Through this expansion, we will be able to fuel a new era of biology, the post-genomic era, where biologists accelerate basic scientific insight and biomedical advances through the power of next-generation protein sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.