In the development of novel drugs for oral administration, it is essential to assess the absorption, metabolism, and excretion of candidate therapeutics using reliable intestinal experimental models.
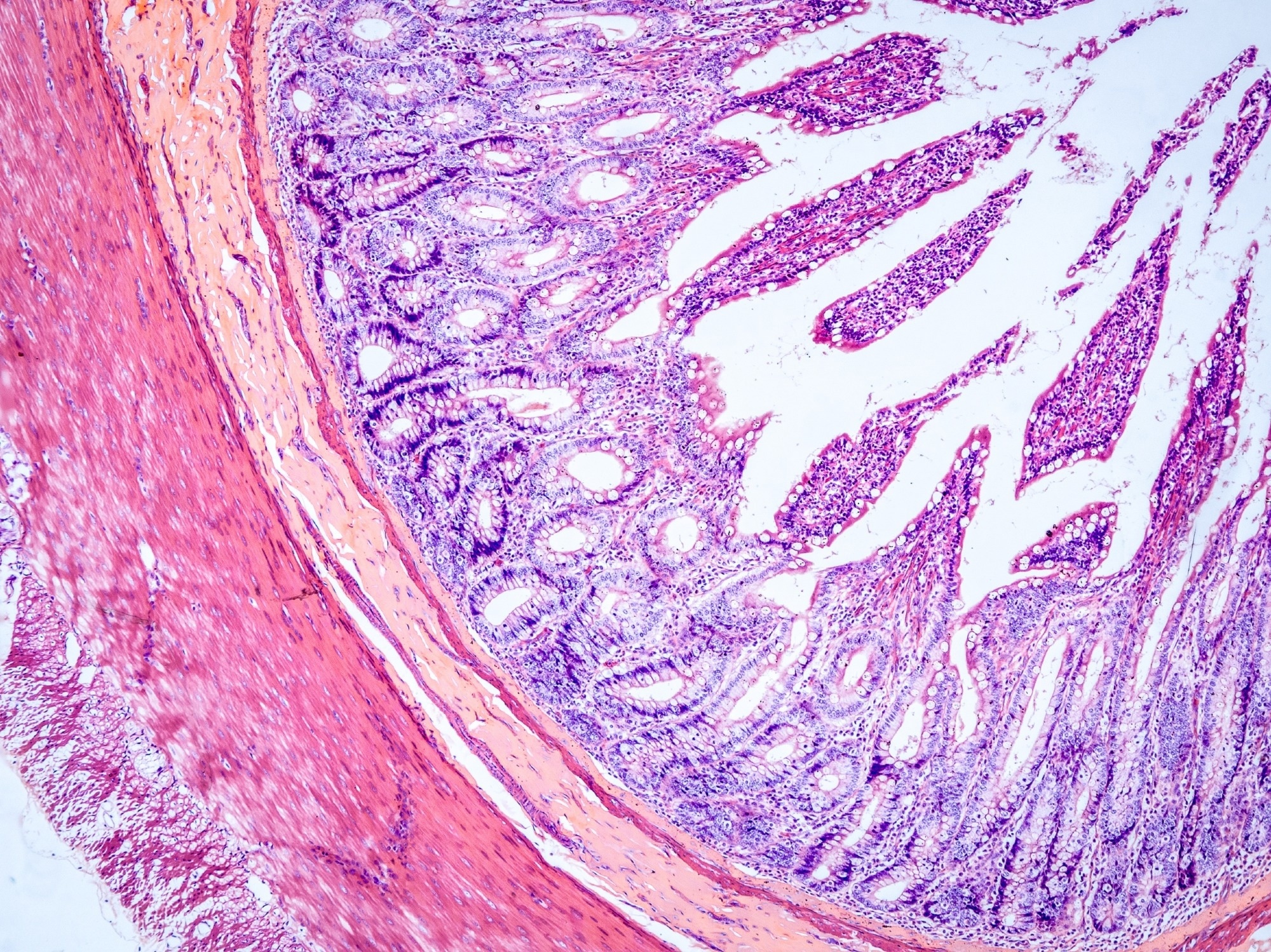
Image Credit: Joan Carles Juarez/Shutterstock.com
While in vivo animal models are commonly employed for pharmacokinetic studies, they are often expensive, low in throughpu,t and typically fail to accurately replicate key characteristics of the human intestine concerning drug metabolism and bioavailability due to species differences.
For several years, human intestinal cancer cell lines cultured as monolayers in vitro have served as an alternative model system. However, despite the availability and ease of expansion of established cancer lines, they lack critical physiological features, including appropriate tissue cytoarchitecture and the expression of transporters and enzymes when compared to normal intestinal tissue.
Organoid-derived intestinal monolayers
The advent of organoid cultures has significantly advanced the modeling of the human intestinal epithelium ex vivo.
However, intestinal organoids are typically cultivated within a hydrogel as three-dimensional (3D) structures with a lumen that is not easily accessible, posing challenges for evaluating the transport of molecules across the epithelium.
Numerous studies have suggested the cultivation of cells derived from intestinal organoids as two-dimensional (2D) monolayers on permeable supports, allowing for the convenient addition of test compounds and sampling of media from either side of the epithelium.
These organoid-derived monolayers offer the opportunity to examine the interactions between drugs and the intestinal barrier ex vivo while addressing major limitations associated with animal models and established tumor cell lines.
Nonetheless, issues regarding accessibility and the availability of human intestinal organoids may hinder their widespread application in the drug development process.
3D ready intestinal organoids
In the study discussed here, epithelial monolayers were derived from two 3D Ready™ non-cancerous human duodenal organoid lines (DP8N3 and DP41N2) from Molecular Devices.
Morphological and functional analyses were performed to evaluate the resulting monolayers for their capacity to model the human intestinal barrier.
Molecular Devices' proprietary bioprocess technology facilitates the expansion of reproducible and validated batches of 3D ready organoids, thereby enabling the easier implementation of relevant in vitro barrier models.
Download the poster
Acknowledgments
Produced from material originally authored by Giusy Tornillo, Harman Chaggar, Kim Luetchford, Kirsty Greenow and Victoria Marsh Durban from Molecular Devices.
About Molecular Devices UK Ltd 
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.