The development of a novel hit identification workflow, integrating an AI-driven virtual screen with advanced biophysical methods, is reported in the article below. Its application to the BRPF1b bromodomain enabled the swift identification of micromolar binders exhibiting favorable in vitro ADMET parameters from a virtual collection of almost 25 million commercial compounds.
Beyond the exceptional hit rate exhibited with BRPF1b, the workflow delivers full binding kinetics and predicts ligand binding topologies without requiring new ligand-protein structures. This accelerates not only hit identification programs but also all subsequent phases of the pharmaceutical discovery pipeline.
A lean and versatile Hit identification process
With more than 200,000 protein structures already deposited in the Protein Data Bank and numerous models generated by Alphafold, structurally enabled virtual screening can now be applied to most biological targets. However, brute-force docking of massive virtual collections remains computationally demanding without access to supercomputers.
Concept Life Sciences overcomes these technical challenges by incorporating cutting-edge AI approaches, including Molecular Pool-based Active Learning (MolPAL), into virtual screening protocols.1
In vitro validation of virtual hits typically requires the development of custom assays for each biological target. To standardize this process and quickly identify optimal binders, a flexible biophysical workflow supported by Grating-Coupled Interferometry was established. Figure 1 outlines this hit identification process for the BRPF1b bromodomain, an attractive oncology target.
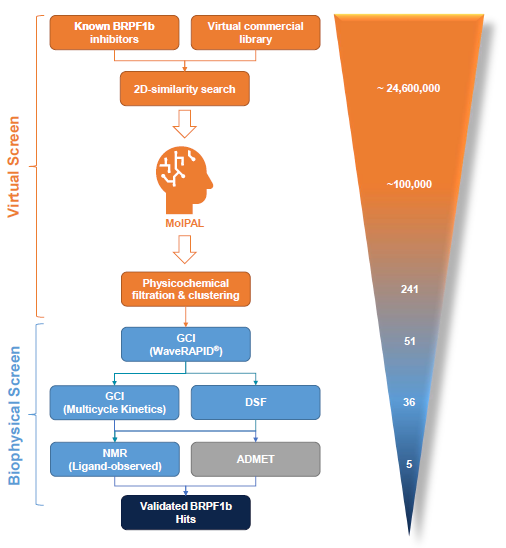
Figure 1. The hit identification process is centred on the MolPAL algorithm and a GCI-driven biophysical hit confirmation workflow. In silico activities are displayed in orange, biophysical screening activities in blue and ADMET profiling in grey. Figures in bracket correspond to the number of compounds processed though each individual stage for the BRPF1b bromodomain. Image Credit: Concept Life Sciences
BRPF1b – an actionable target
Bromodomains are epigenetic proteins that recognize acetylated histone tails. Their activity can lead to chromatin remodelling and influence gene expression that contributes to cancer, inflammation, and neurological disorders.2
Bromodomain and PHD finger-containing protein 1b (BRPF1b) modulates gene transcription through multiple chromatin reader domains, including a double PHD and zinc finger assembly, a bromodomain, and a C-terminal PWWP domain (Figure 2). BRPF1b has been recognized as a promising therapeutic target to treat HCC and AML, an aggressive cancer with a 5-year survival rate below 30 % in adults.3,4,5
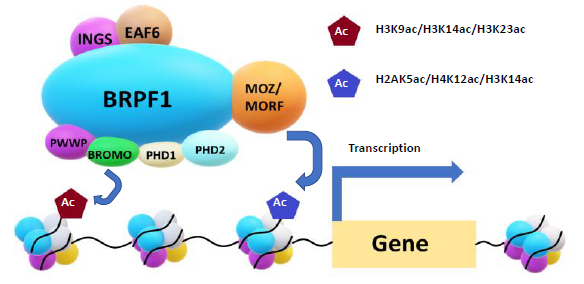
Figure 2. The BRPF1/MOZ/MORF protein-protein interaction complex. Image Credit: Concept Life Sciences
Several BRPF1b bromodomain inhibitors have been reported in recent years, including GSK6853 and NI-57 (Figure 3).6,7 In silico methods, including high-throughput fragment docking and ligand-based screening, have been utilized to identify additional chemotypes, with optimization aided by X-ray crystallography.8,9
Despite these efforts, no selective BRPF1b inhibitor has progressed to clinical evaluation and novel chemotypes inhibiting this target remain in high demand.
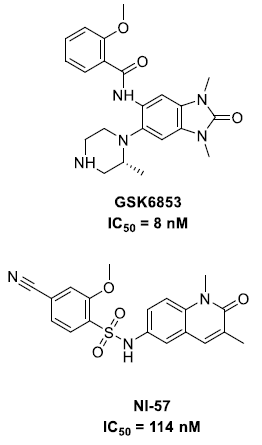
Figure 3. BRPF1b inhibitors. Image Credit: Concept Life Sciences
AI-driven virtual screening - MolPAL
The MolPAL structure-based virtual screening algorithm (Figure 4) predicts docking scores from simple molecular fingerprints. High-ranking compounds are readily detected even though MolPAL docks only a fraction (typically ~1%) of the entire compound collection.1
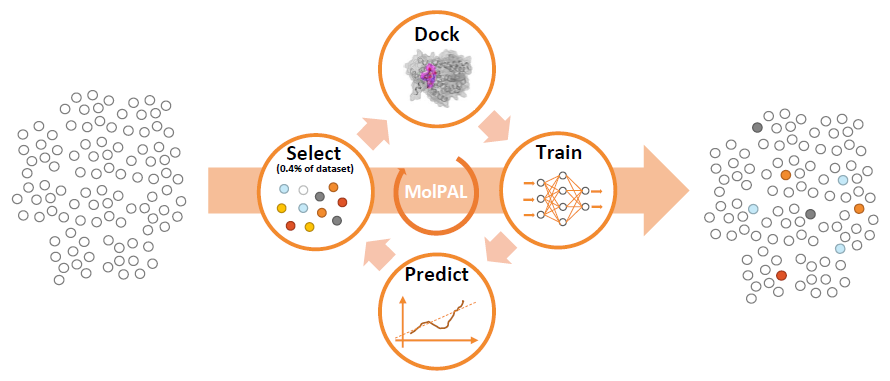
Figure 4. Schematic representation of the MolPAL protocol developed and reported by Graff and coworkers.1 Image Credit: Concept Life Sciences
After an initial 2D-similarity search of 24.6 million commercial compounds, approximately 100,000 compounds were subjected to MolPAL (4 cycles) through a cloud computing platform. Subsequent cheminformatic triage of the top virtual hits yielded 51 diverse, lead-like virtual hits with binding topologies as predicted by MolPAL (Figure 5).
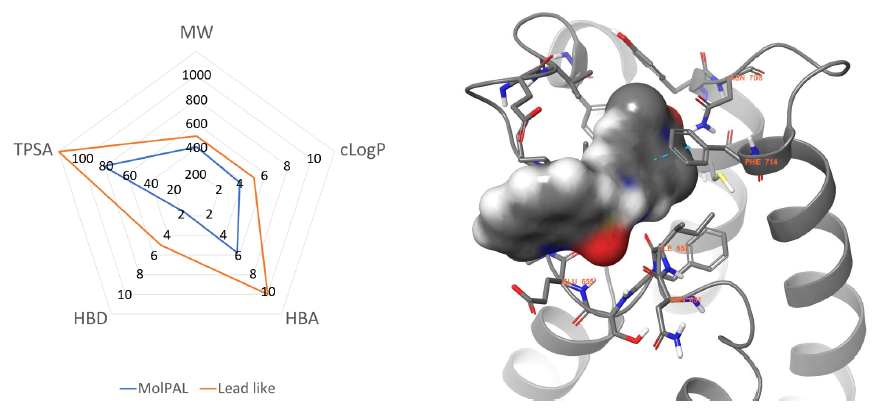
Figure 5. Left: Physicochemical profile of the MolPAL output (blue) compared to lead-like boundaries (orange). Right: Lowest energy docking pose predicted by MolPAL for compound 5 (grey surface) within the BRPF1b active site (grey cartoon, PDB = 6EQK).9 Key residues are represented in grey sticks and amino acid sequence numbers with orange labels. Nitrogen, Oxygen and Sulfur atoms are coloured blue, red and yellow respectively. π- π interactions are represented with dotted cyan lines. Image Credit: Concept Life Sciences
Primary in vitro confirmation - GCI
Grating-coupled interferometry (GCI, Figure 6) is a label-free biosensing method that provides exceptional resolution of ultrafast binding kinetics, usually observed during the early phases of drug discovery, such as hit identification.10 WaveRAPID® (Repeated Analyte Pulses of Increasing Duration) injects a single concentration, pulsing the sample across the sensing surface at increasing durations.10
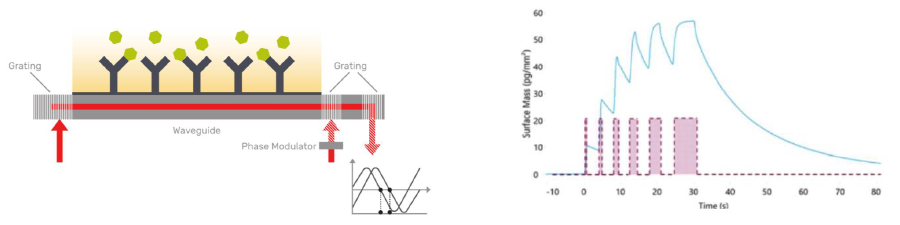
Figure 6. Left: Schematic representation of the GCI technology. Analyte (green) is run over a biomolecule (black) immobilised on a sensor chip (grey). Binding is measured across the sensor chip through changes in the refractive index of the evanescent field near the sensing surface (red). Right: waveRAPID® pulsing technology. Image Credit: Concept Life Sciences
WaveRAPID® GCI screening of the 51 virtual hits (100 μM) identified 36 primary hit binders (Figure 7). Multicycle kinetics validated 20 compounds bound BRPF1b with KD < 250 μM (39% hit rate). Binding kinetics were determined for all hits, GSK6853 and NI-57 (Table 1). A BRPF1a counter-screen was conducted, with no binders detected for this target, demonstrating hit selectivity for BRPF1b over BRPF1a.
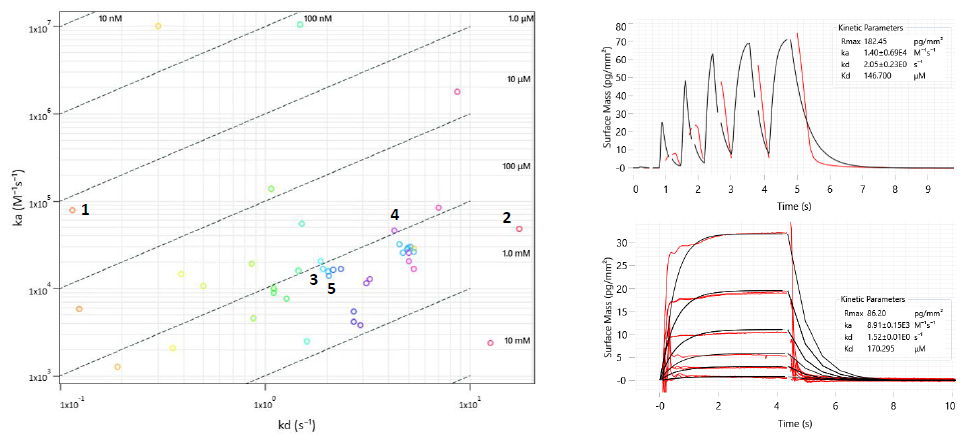
Figure 7. Left: Two-dimensional isoaffinity kinetic plot of association (ka) and dissociation (kd) rate constants from WaveRAPID® screen of 51 virtual hits. The 36 primary hit binders are shown. Diagonal lines indicate equilibrium binding constants (KD). Each circle represents a binder and is coloured according to the dissociation rate. Top right: WaveRAPID® GCI sensorgram of compound 5 (100 μM) binding to BRPF1b. Bottom right: Multicycle kinetics GCI sensorgram of 5 binding to BRPF1b. Image Credit: Concept Life Sciences
Orthogonal in vitro confirmation - DSF
The 36 WaveRAPID® hits were orthogonally verified using differential scanning fluorimetry (DSF) at 100 μM. Thirteen compounds induced a BRPF1b thermal shift (ΔTm) exceeding ± 4 °C, indicating a strong interaction with the protein (Figure 8). Four hits produced a thermal shift equal to or greater than GSK6853 and NI-57.
Eight hits showing both KD < 250 μM and ΔTm > ±4° C were detected, including compounds 1, 2, 3, 4, and 5 (Table 1). This provided a prioritized list of BRPF1b hits verified in vitro through a combination of orthogonal methods (GCI and DSF).
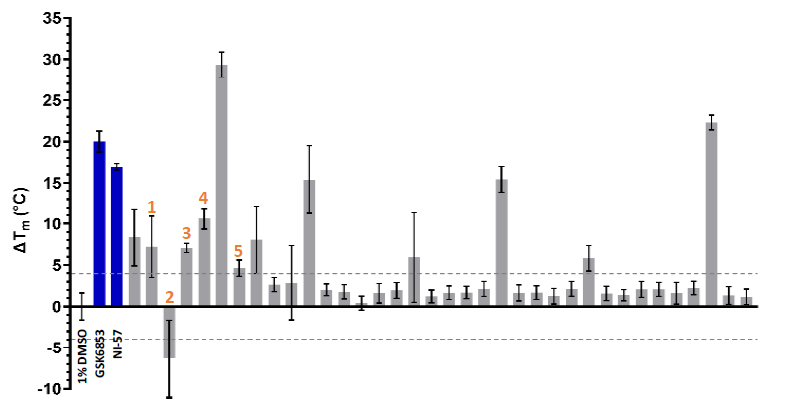
Figure 8. DSF characterization of the of 36 waveRAPID® primary hits (grey). GSK6853 and NI-57 are coloured in blue, and the > +4 °C and < -4 °C thresholds are indicated with grey dashed lines. Image Credit: Concept Life Sciences
Hit binding topology – Ligand-observed NMR
Binding was further verified for 4 of the 5 prioritized hits utilizing various ligand-observed NMR acquisition approaches, including 1D, STD, waterLOGSY, and CPMG, in both the presence and absence of BRPF1b protein.11,12,13,14 For several ligands, hit binding topologies were inferred based on specific shift changes upon protein addition (Figure 9), verifying MolPAL-predicted docking poses.
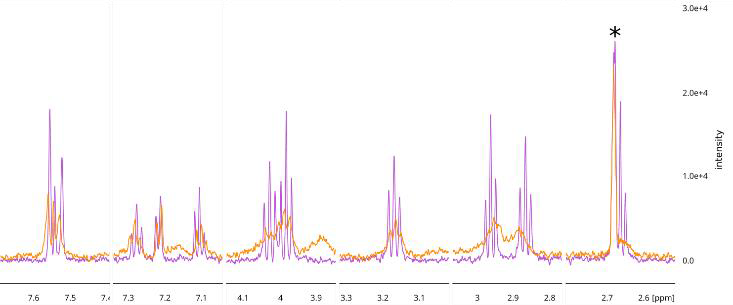
Figure 9. Sections of the 1D 1H NMR spectra illustrating peaks from compound 5 (400 μM) in the absence (pink) and presence (orange) of the BRPF1b bromodomain (26 μM). *Denotes a peak from a contaminant component of the sample. Image Credit: Concept Life Sciences
Table 1. Summary of biophysical data for GSK6853, NI-57 and five BRPF1b hits. cLogDs were determined using QikProp. KD, ka and kd were determined by GCI multicycle kinetics (N=2). ΔTm was determined by DSF (N=2). Ligand-observed NMR binding classification results from a consensus using 1D, STD, waterLOGSY and CPMG acquisition methods. n.d. = not determined. Source: Concept Life Sciences
| Entry |
MW |
cLogD |
KD |
ka |
kd |
ΔTm |
NMR binding |
| unit |
g.mol-1 |
- |
μM |
M.s-1 |
s-1 |
°C |
- |
| GSK6853 |
409 |
2.9 |
0.078 |
1.3 x 106 |
0.01 |
+ 19.9 |
n.d. |
| NI-57 |
383 |
1.5 |
0.086 |
1.9 x 107 |
0.16 |
+ 17.0 |
n.d. |
| 1 |
448 |
3.9 |
44 |
4.6 x 104 |
2.0 |
+ 7.2 |
Yes |
| 2 |
431 |
2.8 |
46 |
4.8 x 104 |
2.2 |
- 6.3 |
Yes |
| 3 |
367 |
2.1 |
61 |
3.5 x 104 |
2.0 |
+ 7.1 |
Insoluble |
| 4 |
369 |
2.9 |
128 |
3.0 x 104 |
2.1 |
+ 10.6 |
Yes |
| 5 |
354 |
2.1 |
186 |
8.4 x 103 |
1.6 |
+ 4.6 |
Yes |
Selecting the optimal hits – ADMET profiling
ADMET profiling of the 5 prioritized hits demonstrated that compounds 1, 2, 3, and 4 are metabolically stable in human microsomes and hepatocytes (Table 2). Further CYP450 characterization of compounds 1 and 2 provided further evidence of their potential for development to treat poly-medicated cancer patients.
Table 2. In vitro ADMET profile of five BRPF1b hits. CYP450 inhibition was measured as a pooled mixture of 1A2, 2C9 2C19, 2D6 and 3A4 isoforms. MPPB = mouse plasma protein binding, MLM = mouse liver microsomal stability, RLM = rat liver microsomal stability, HLM = human liver microsomal stability, HHeps = human hepatocyte stability, Fu = fraction unbound, n.d. = not determined. Source: Concept Life Sciences
| Entry |
MPPB
(Fu) |
MLM
(Clint) |
RLM
(Clint) |
HLM
(Clint) |
HHeps
(Clint) |
CYP450
inhib. (IC50) |
| unit |
- |
μL.min-1.mg-1 |
μL.min-1.mg-1 |
μL.min-1.mg-1 |
μL.min-1.106 cells |
μM |
| 1 |
0.18 |
17 |
41 |
< 5 |
< 3 |
All > 50 |
| 2 |
0.12 |
< 5 |
< 5 |
< 5 |
< 3 |
All > 10 |
| 3 |
0.23 |
20 |
89 |
10 |
4 |
n.d. |
| 4 |
0.12 |
6 |
< 5 |
< 5 |
< 3 |
n.d. |
| 5 |
0.05 |
157 |
216 |
94 |
67 |
n.d. |
Summary
A streamlined and versatile hit identification workflow was developed by combining innovative AI and biophysical methodologies. This process revealed 51 in silico hits from more than 24 million commercial compounds. Thirty-six were verified in vitro as primary binders using GCI, including 20 with KD < 250 μM. Four orthogonally validated hits provide strong starting points for the creation of novel BPRF1b inhibitors.
Acknowledgments
Produced from materials originally authored by Thomas Pesnot, Sandeep Pal, Zandile Nare Vincenzo A. Rao, Ian Morrison, Andrew Scott, Matilda J. Bingham, Muhammad Jan, Brian O. Smith and Edward A. Fitzgerald.
References and further reading:
- Graff, D.E., Shakhnovich, E.I. and Coley, C.W. (2021). Accelerating high-throughput virtual screening through molecular pool-based active learning. Chemical Science, 12(22), pp.7866–7881. https://doi.org/10.1039/d0sc06805e.
- You, L., et al. (2015). Deficiency of the Chromatin Regulator Brpf1 Causes Abnormal Brain Development. Journal of Biological Chemistry, 290(11), pp.7114–7129. https://doi.org/10.1074/jbc.m114.635250.
- Cheng, C.L.-H., et al. (2021). Bromodomain-containing protein BRPF1 is a therapeutic target for liver cancer. Communications Biology, 4(1). https://doi.org/10.1038/s42003-021-02405-6.
- Shima, H., et al. (2013). Bromodomain-PHD finger protein 1 is critical for leukemogenesis associated with MOZ–TIF2 fusion. International Journal of Hematology, [online] 99(1), pp.21–31. https://doi.org/10.1007/s12185-013-1466-x.
- Cancer Research UK (2016). Acute myeloid leukaemia (AML) | Cancer Research UK. [online] Cancerresearchuk.org. Available at: https://www.cancerresearchuk.org/about-cancer/acute-myeloid-leukaemia-aml.
- Bamborough, P., et al. (2016). GSK6853, a Chemical Probe for Inhibition of the BRPF1 Bromodomain. ACS Medicinal Chemistry Letters, 7(6), 552–557. https://doi.org/10.1021/acsmedchemlett.6b00092
- Igoe, N., et al. (2017). Design of a Chemical Probe for the Bromodomain and Plant Homeodomain Finger-Containing (BRPF) Family of Proteins. Journal of Medicinal Chemistry, 60(16), pp.6998–7011. https://doi.org/10.1021/acs.jmedchem.7b00611.
- Zhu, J. and Caflisch, A. (2016). Twenty Crystal Structures of Bromodomain and PHD Finger Containing Protein 1 (BRPF1)/Ligand Complexes Reveal Conserved Binding Motifs and Rare Interactions. Journal of Medicinal Chemistry, 59(11), pp.5555–5561. https://doi.org/10.1021/acs.jmedchem.6b00215.
- Zhu, J., Zhou, C. and Caflisch, A. (2018). Structure-based discovery of selective BRPF1 bromodomain inhibitors. European Journal of Medicinal Chemistry, 155, pp.337–352. https://doi.org/10.1016/j.ejmech.2018.05.037.
- Kartal, Ö., et al. (2021). waveRAPID—A Robust Assay for High-Throughput Kinetic Screens with the Creoptix WAVEsystem. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 26(8), pp.995–1003. https://doi.org/10.1177/24725552211013827.
- Hajduk, P.J., Olejniczak, E.T. and Fesik, S.W. (1997). One-Dimensional Relaxation- and Diffusion-Edited NMR Methods for Screening Compounds That Bind to Macromolecules. Journal of the American Chemical Society, 119(50), pp.12257–12261. https://doi.org/10.1021/ja9715962.
- Mayer, M. and Meyer, B. (1999). Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angewandte Chemie International Edition, 38(12), pp.1784–1788. https://doi.org/10.1002/(sici)1521-3773(19990614)38:12%3C1784::aid-anie1784%3E3.0.co;2-q.
- Dalvit, C., et al. (2001). Journal of Biomolecular NMR, 21(4), pp.349–359. https://doi.org/10.1023/a:1013302231549.
- Larsen, F.H., et al. (1997). Sensitivity-Enhanced Quadrupolar-Echo NMR of Half-Integer Quadrupolar Nuclei. Magnitudes and Relative Orientation of Chemical Shielding and Quadrupolar Coupling Tensors. The Journal of Physical Chemistry A, 101(46), pp.8597–8606. https://doi.org/10.1021/jp971547b.
About Concept Life Sciences
Concept Life Sciences is a leading contract research organisation (CRO) serving the global life sciences industry. For over 25 years, the company, and its heritage companies, have provided consultative and collaborative drug discovery and development services. Our approach, supported by passionate scientists and world-leading capabilities, enables clients to overcome complex scientific challenges across a broad range of therapeutic areas, improving program success rates. The company has successfully helped 29 candidates advance to the clinic.
The company offers sophisticated translational biology services coupled with exceptional end-to-end chemistry capabilities across all modalities, including small molecules, biologics, peptides and cell & gene therapies, with the ability to seamlessly integrate capabilities and provide bespoke solutions to address client needs.
Collectively, the company’s high-quality services and commitment to customer service across the drug development pathway enhance efficiency in drug discovery, helping clients advance their drugs to clinic in as little as 32 months, well ahead of the industry average of 60 months.
Driven by a passion for science, Concept Life Sciences has around 230 employees, with around 70% holding PhDs. The company operates from state-of-the-art UK facilities, headquartered near Manchester, with additional operations in Edinburgh, Dundee, and Sandwich. The headquarters is one of the UK’s largest medicinal chemistry CRO sites with key discovery services all under one roof.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.