Reducing the time and expense of engineering PROTAC degraders remains a significant challenge in the pharmaceutical industry. A PROTAC is composed of an E3 ligase ligand (E3-L), a protein of interest ligand (POI-L), and a linker. PROTAC efficiency depends on the formation of a ternary complex with the E3 ligase and POI, with linker optimization playing a crucial role in improving potency, cooperativity, and physicochemical attributes.
Nevertheless, the large size and complex design of PROTACs make optimization time-intensive, particularly when relying on cell-based assays. This research applied a Direct-to-Biology (D2B) approach, merging automated chemistry with the screening of crude reaction mixtures. This high-throughput technique enabled rapid examination of linker intermediates, offering a time- and cost-efficient path to PROTAC optimization.
Heterobifunctional degraders hit finding using D2B platform
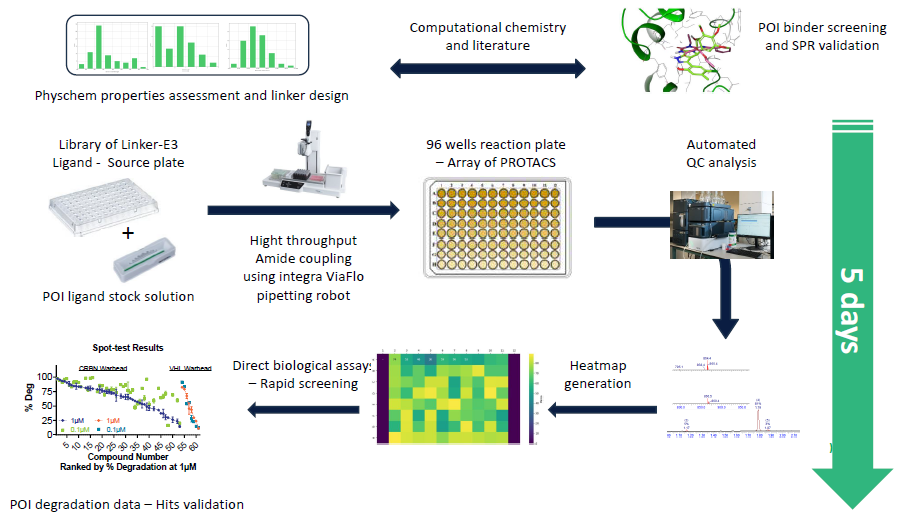
Figure 1. Overview of the automated D2B platform developed at Charnwood allowing high-throughput PROTAC hit finding and rapid SAR generation. Image Credit: Concept Life Sciences
D2B is a semi-automated process that allows fast biological assessment of crude reaction mixtures within a plate, shortening the PROTAC optimization cycle to just five days.
Heterobifunctional degraders synthesis using plate-based chemistry
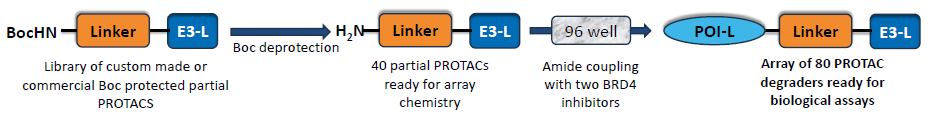
Figure 2. Our approach for high throughput plate-based chemistry for direct biological assays. Image Credit: Concept Life Sciences
BRD4 was chosen as a model target to develop the methodology. Two novel BRD4 inhibitors were produced and coupled to a series of E3-Ligand-Linker via an amide coupling reaction (Figure 2). Amide coupling proved to be a high-yielding reaction as demonstrated by the heatmap produced with PyParse (Figure 3C).
Figure 3B highlights the advantages of utilizing the D2B method compared to the traditional procedure, which is significantly more time- and cost-intensive. Shape, length, attachment point to the E3-L, and physicochemical attributes of the linker are crucial design factors for potent and selective PROTAC degraders. Table 1 outlines the approach and systematic design for the linker intermediates library.
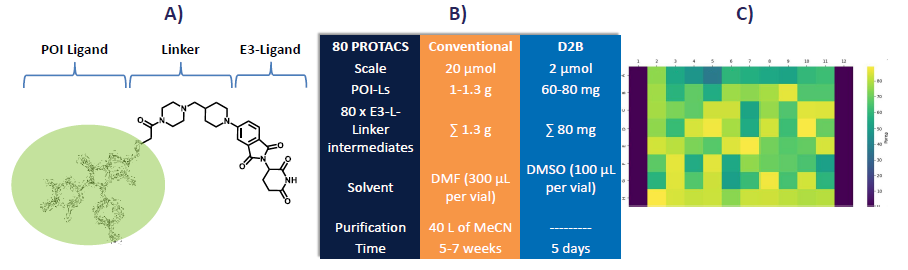
Figure 3. A) Example structure of a PROTAC molecule. B) Comparison of conventional synthesis (purified) and D2B (non-purified) workflow for a theoretical 80 PROTACS library. C) Heatmap for reaction conversion generated using PyParse. Image Credit: Concept Life Sciences
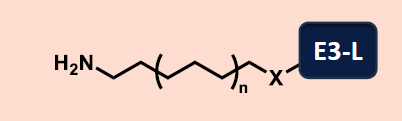
Table 1. Different categories of E3-L-Linker intermediates within our library. Source: Concept Life Sciences
| Aliphatic and PEG linkers |
Rigidified linkers |
Bespoke linkers |
 |
 |
 |
- Linker length/attachment point exploration
- Limited ADME/DMPK advantages
|
- Systematic exit vector exploration with thalidomide E3 ligase ligand
- Improved ADME/DMPK properties
|
- Bespoke singletons from internal and literature
- Improved selectivity and/or ADME/DMPK properties
|
Identifying novel inhibitors of BRD4
Virtual screening of Enamine's drug-like library for BRD4 BD1 hits (Figure 4A) revealed 30 candidate compounds for additional profiling. These compounds were initially assessed for activity utilizing an SPR assay (Figure 4B), followed by confirmation in a cell-based assay, depicted in an LLE plot (Figure 4C) and an FP assay (data not shown). Docking the novel compound into the active site’s crystal structure identified two exit vectors to guide PROTAC development (Figure 4D).
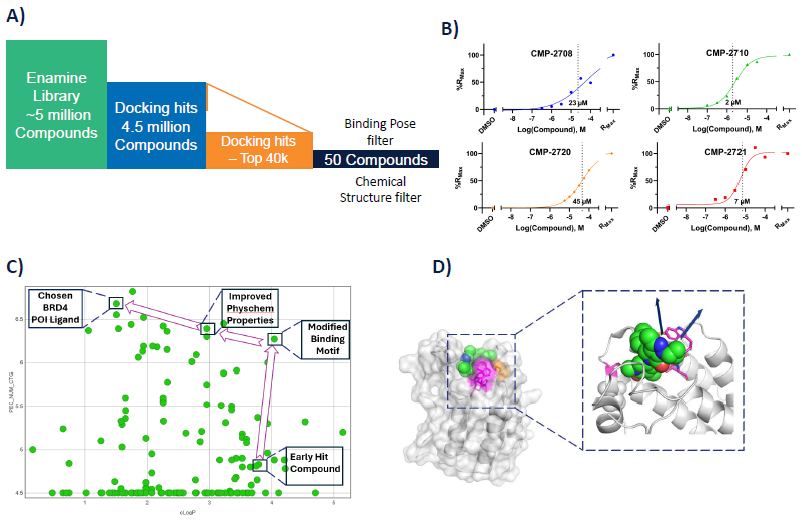
Figure 4. A) High level overview of virtual screening. B) SPR data for hit compounds. C) LLE plot and SAR development using a BRD4 dependant MCF7 cell line. D) Docked image of our hit molecule using pdb: 6ZCI, exemplifying two potential exit vectors. Image Credit: Concept Life Sciences
Biological results
Crude compound mixtures were screened in a BRD4-HiBiT degradation assay. From a 96-well plate stock, liquid handlers diluted the crude compound mixture into 384-well cell plates at 1 μM and 0.1 μM concentrations (Figure 5A). This technique minimized false negatives from the hook effect, a frequent observation with PROTACs. Compounds of interest were developed, and a full dose-response assay was conducted to profile degradation potential in parallel with purified batches (Figure 5B).
No major potency variations were observed between crude and purified compounds, as verified by BRD4-HiBiT degradation (Figure 5B) and JESS Western Blot when probed with a BRD4 antibody (Figure 5C). Initial screening identified PROTACs with picomolar DC50 values. The DMPK team performed additional ADME profiling to evaluate essential physicochemical attributes (Figure 5D).
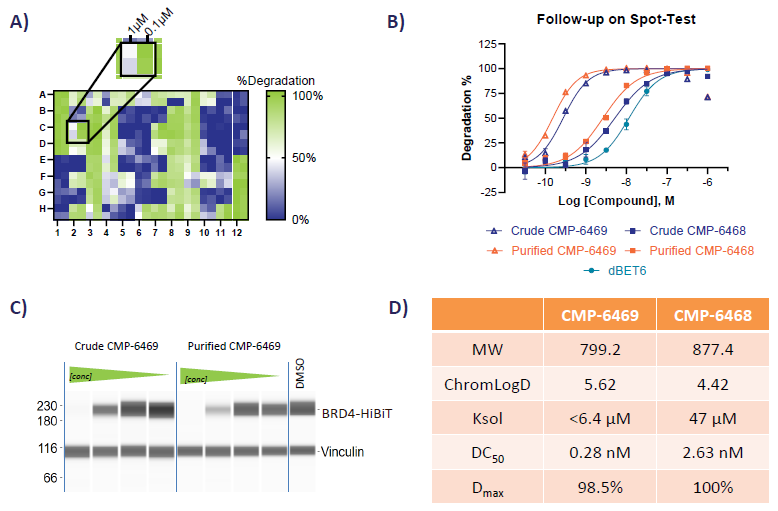
Figure 5. A) Heat map of % degradation data B) Full Dose-response of some of the Hits identified. C) CMP-6469, assayed through JESS WB D) Table showing some of the PhysChem properties of the PROTACs screened. Image Credit: Concept Life Sciences
Summary
The study demonstrates that PROTAC discovery can be accelerated through the D2B method, decreasing hit-finding time from multiple months to five days. Charnwood Discovery's computational design capabilities facilitated the identification of highly potent BRD4 inhibitors.
The D2B platform, including plate chemistry and high throughput in cell assay validation, was also validated. An in-house library is available immediately for reaction with POI-Ligands to support integrated pharmaceutical discovery projects.
Acknowledgments
Produced from materials originally authored by Hassan Srour, Joshua McArthur, Charles Simon, Bin Zhou, Samual Adams, Anthony Ball, Ryan Mordue, Sean Smith, Peter Breed, Sarah Mistry, Victoria Ford, Lucy Burton, Craig Avery, James Hitchin, Kathy Dodgson, Ralph Kirk Charnwood Discovery, a Concept Life Sciences company,.
References and further reading
- Hendrick, C.E., et al. (2022). Direct-to-Biology Accelerates PROTAC Synthesis and the Evaluation of Linker Effects on Permeability and Degradation. ACS Medicinal Chemistry Letters, 13(7), pp.1182–1190. https://doi.org/10.1021/acsmedchemlett.2c00124.
- Stevens, R., et al. (2023). Integrated Direct-to-Biology Platform for the Nanoscale Synthesis and Biological Evaluation of PROTACs. Journal of Medicinal Chemistry, 66(22), pp.15437–15452. https://doi.org/10.1021/acs.jmedchem.3c01604.
- Plesniak, M.P., et al. (2023). Rapid PROTAC Discovery Platform: Nanomole-Scale Array Synthesis and Direct Screening of Reaction Mixtures. ACS medicinal chemistry letters, 14(12), pp.1882–1890. https://doi.org/10.1021/acsmedchemlett.3c00314.
About Concept Life Sciences
Concept Life Sciences is a leading contract research organisation (CRO) serving the global life sciences industry. For over 25 years, the company, and its heritage companies, have provided consultative and collaborative drug discovery and development services. Our approach, supported by passionate scientists and world-leading capabilities, enables clients to overcome complex scientific challenges across a broad range of therapeutic areas, improving program success rates. The company has successfully helped 29 candidates advance to the clinic.
The company offers sophisticated translational biology services coupled with exceptional end-to-end chemistry capabilities across all modalities, including small molecules, biologics, peptides and cell & gene therapies, with the ability to seamlessly integrate capabilities and provide bespoke solutions to address client needs.
Collectively, the company’s high-quality services and commitment to customer service across the drug development pathway enhance efficiency in drug discovery, helping clients advance their drugs to clinic in as little as 32 months, well ahead of the industry average of 60 months.
Driven by a passion for science, Concept Life Sciences has around 230 employees, with around 70% holding PhDs. The company operates from state-of-the-art UK facilities, headquartered near Manchester, with additional operations in Edinburgh, Dundee, and Sandwich. The headquarters is one of the UK’s largest medicinal chemistry CRO sites with key discovery services all under one roof.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.