Concept Life Sciences recently showcased its ability to rapidly transfer a client process into its own GMP facilities, enabling the manufacture of 1.5 kg of API to support early-phase clinical trials.
This transfer involved the implementation of a complex eight-step synthesis process featuring novel chemistry, harsh and hazardous reaction conditions, and a final API crystallization that was highly challenging from a technical perspective.
Concept Life Sciences was able to accelerate this program from Hit to Lead (H2L) to candidate nomination within an 18-month period, with API and formulated drug product characterization key to facilitating fast-tracked final selection. Early de-risking of the process also enabled the development, manufacture, and release of the API, allowing phase I clinical trials to begin.
Project overview
The goal of this project was to develop a functionalization strategy for a complex heterocycle backbone in two positions. A total of 300 compounds were synthesized and tested for ADMET and other specific relevant biological assays.
Image Credit: Concept Life Sciences
This process involved:
- Route scouting to remove any challenging fluorination chemistry
- Process development and optimization for efficient scale-up
- The determination and evaluation of essential process parameters
- Managing the stability and composition of processing streams
- Developing analytical methods able to define IPC criteria, better understand impurities, and support the setting of release specifications
- Testing process safety and evaluating hazards
- Development of an appropriate API ICH stability program
Process development
The HEL PolyBlock 4 platform was employed to rapidly assess process variations, characterize key parameters, and perform precise liquid additions alongside controlled heating and cooling. This enabled the team to identify how raw material particle size influenced critical heterogeneous coupling reactions, thanks to the system’s ability to deliver highly repeatable mixing conditions.
A Design of Experiments (DoE) approach was then applied to optimize intermediate synthesis steps, leading to higher yields and improved purity—both essential for robust GMP manufacture.
During development, it was discovered that crystallization was being inhibited by an unidentified impurity. By modifying conditions with co-solvent addition and seeding, a more reliable and repeatable crystallization process was established.
In parallel, the approach also provided the opportunity to explore the application of flow chemistry for a rapid, highly exothermic reaction step within the intermediate synthesis, further demonstrating the flexibility of the process development strategy.

HEL PolyBlock 4. Image Credit: Concept Life Sciences
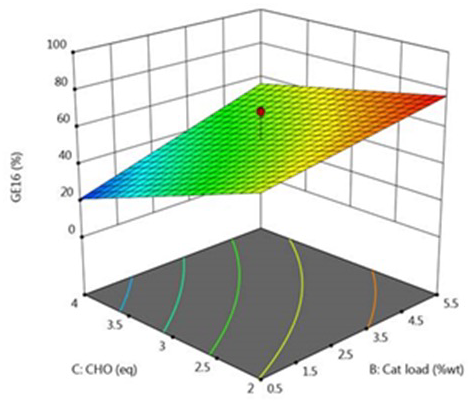
DoE – 3D plot for interaction BC (% yield). Image Credit: Concept Life Sciences
Process safety
Before implementing GMP manufacture, it was necessary to complete a full process hazard assessment incorporating process safety testing to ensure that every aspect of process safety was effectively captured and managed.
A number of reactions/additions in the synthesis relied on the use of DSC, TSU, and reaction calorimetry to better understand and assess gas evolution and exothermic reaction hazards.

HEL Thermal Screening Unit. Image Credit: Concept Life Sciences
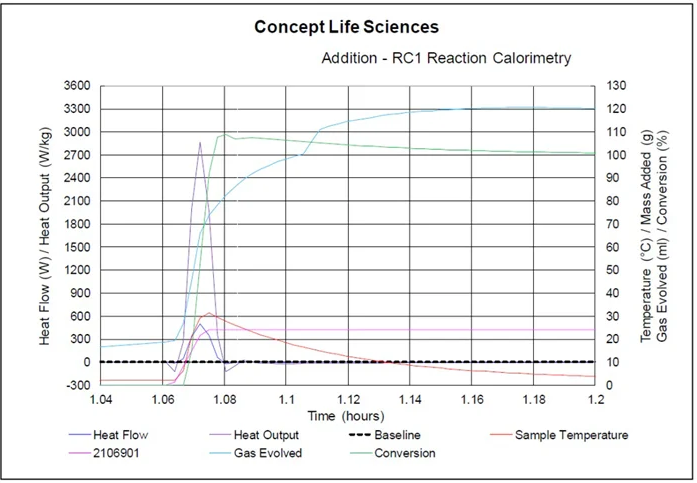
RC1 Reaction Calorimetry plot. Image Credit: Concept Life Sciences
A combination of RC1 and TSU testing provided invaluable data and enabled an improved understanding of adiabatic temperature rises, gas generation, and reactant accumulation. This was key to determining a series of safe operating parameters for the process.
Comprehensive process safety data is essential for ensuring appropriate plant engineering controls during ongoing process scale-up. Key data is also required to perform a Hazard and Operability Assessment (HAZOP).
Manufacture
A 20 L Büchi jacketed Hastelloy GMP vessel was selected for key transformation to remove the risk of in-situ HF generation during the reaction. Hastelloy offers excellent corrosion resistance and is able to accommodate a wide range of reagents and process conditions.
The vessel setup employed in this instance mirrors that of a typical pilot- or commercial-scale reactor, allowing seamless transition from kilo to pilot scale. A 60 l jacketed Büchi GMP vessel was also used for larger volume steps and final API crystallization.
This vessel can accommodate a wide temperature range from -90 °C to 250 °C and is fitted with a Huber 161 W thermoregulation unit to facilitate precise temperature control.

20 L Büchi jacketed Hastelloy vessel. Image Credit: Concept Life Sciences

Image Credit: Concept Life Sciences
It is also possible to easily modify the vessel to enable in-process monitoring or the installation of sampling equipment. The addition of a separate, smaller header vessel allowed for the straightforward manipulation and charging of highly air-sensitive reagents, while the connection of the vessel to a contained filter allowed for the safe transfer and isolation of hazardous intermediates.
Key impurities were identified and trended within the process to collect additional data for the client. The client was also provided with frequent project updates via a multidisciplinary team with support from a dedicated program manager.
Technical Transfer Protocols (TTPs) were generated for the client. These protocols incorporated key developmental changes to the process, existing process knowledge, and information on any new understanding acquired during kilo scale manufacture.
Analytical and release testing
Test method transfer of in-process control (IPC) methods was performed. This included testing of registered starting materials (RSMs), raw materials, and intermediates. Release testing was coordinated by the quality team.
Analytical methods were developed and validated to support the setting of specifications and the release of the final API. This involved a wide range of analytical techniques, including:
- HPLC/UPLC
- Headspace Gas Chromatography (GC-HS)
- X-ray powder diffraction (XRPD)
- Inductively coupled plasma-optical emission spectrometry (ICP-OES)
- Ion Chromatography (IC)
- Particle size distribution (PSD)
A cleaning verification method was also developed to ensure that manufacturing equipment was effectively cleaned following API manufacture.

Image Credit: Concept Life Sciences
Summary
This project enabled accelerated H2L to candidate nomination through the acquisition of robust analytical data design to support decision-making.
Route scouting was successful, with DoE experiments helping to improve yields and purity profiles, while effective process development and optimization enabled the delivery of chromatography-free, convergent synthesis.
The comprehensive gathering of process safety data allowed for improved confidence in route selection, supporting the determination of necessary engineering controls.
The manufacturing process leveraged specialist-validated processing equipment that was highly representative of both the pilot and commercial plant setups. Equipment was also de-risked to avoid common issues encountered during the transfer from lab-style glassware at an earlier stage, for example, issues around mass transfer, changes in reaction kinetics, material properties, or issues with fluid and thermodynamics.
This work resulted in the full, on-time delivery of the API to the client while ensuring this was within the agreed quality specification.
Acknowledgments
Based on materials originally authored by Michael Stanley, Jonathan Moore, Julie Jones, Kim James, Felice Daverio, and David Fengas.
About Concept Life Sciences
Concept Life Sciences is a leading contract research organisation (CRO) serving the global life sciences industry. For over 25 years, the company, and its heritage companies, have provided consultative and collaborative drug discovery and development services. Our approach, supported by passionate scientists and world-leading capabilities, enables clients to overcome complex scientific challenges across a broad range of therapeutic areas, improving program success rates. The company has successfully helped 29 candidates advance to the clinic.
The company offers sophisticated translational biology services coupled with exceptional end-to-end chemistry capabilities across all modalities, including small molecules, biologics, peptides and cell & gene therapies, with the ability to seamlessly integrate capabilities and provide bespoke solutions to address client needs.
Collectively, the company’s high-quality services and commitment to customer service across the drug development pathway enhance efficiency in drug discovery, helping clients advance their drugs to clinic in as little as 32 months, well ahead of the industry average of 60 months.
Driven by a passion for science, Concept Life Sciences has around 230 employees, with around 70% holding PhDs. The company operates from state-of-the-art UK facilities, headquartered near Manchester, with additional operations in Edinburgh, Dundee, and Sandwich. The headquarters is one of the UK’s largest medicinal chemistry CRO sites with key discovery services all under one roof.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.