Sponsored Content by AbselionReviewed by Olivia FrostNov 27 2025
Adeno-associated virus (AAV) vectors are a key component of gene therapy, recognized for their safety, extensive targeting capabilities, and versatility in various therapeutic applications.
As development progresses from research to process refinement, accurate quantification of total AAV capsid concentration is critical for ensuring consistency and facilitating informed decision-making. Traditional approaches, such as ELISA, can be time-consuming, serotype-dependent, and sensitive to sample complexity.
The Amperia™ platform uses a sandwich format assay with high-affinity antibodies from Thermo Fisher Scientific to measure multiple AAV serotypes with reliable results across all sample types. Amperia's sensor-based detection and quick turnaround ensure reliable results in day-to-day workflows.
Assay format and workflow
Amperia™ quantifies AAV capsids through a sandwich assay method. The sensor surface contains anti-AAV capture sites, which are normally produced through streptavidin-biotin interaction.
AAV particles in the sample bind to the immobilized capture antibody, followed by the addition of a labeled detection antibody specific to the AAV capsid. As AAV concentrations rise, more detection antibody is maintained on the sensor surface, resulting in a higher signal.
This immediate signal response enables reliable quantification of a variety of sample types, including clarified lysates and crude supernatants.
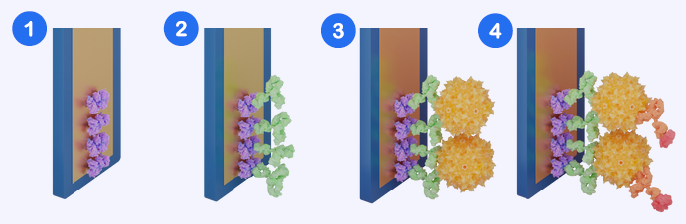
Assay Workflow Schematic. (1) Sensor surface with streptavidin coating. (2) Capture antibody binding via biotin-streptavidin interaction. (3) AAV capsid captured by the immobilized antibody. (4) Detection antibody (HRP-conjugated) binds the captured capsid, enabling signal generation. Image Credit: Abselion
Assays are performed using the system's touchscreen-guided workflow and protocol templates adapted to varied sample sizes. Amperia's straightforward setup and integrated analysis allow for consistent AAV titer determination without the complexities of standard immunoassays.
Source: Abselion
| Parameter |
Typical Value |
| Sample throughput |
Up to 40 samples per run |
| Total run time |
~ 120 min |
| Hands-on time |
~ 15 - 30 min |
| Detection range |
0.78e9 - 1e11 vp/ml |
| Serotypes |
AAV1 - AAV8, AAVrh10*; AAV9 |
| Format |
Sandwich |
*AAVX Kit (AK-AAV-002) validated on AAV2, AAV5, AAV6, AAV8. AAV9 supported via dedicated kit (AK-AAV-003)
Note: Values shown are typical ranges based on internal testing. Actual throughput and hands-on time may vary depending on assay format, sample type, and workflow configuration. Detection range may vary depending on analyte affinity and assay conditions.
Data highlights
Amperia™ provides reliable and reproducible measurements for numerous AAV serotypes.
The AAVX Total Capsid Quantification Kit is designed for broad serotype compatibility, whilst the AAV9-specific kit allows for precise detection of AAV9 particles. Both kits are compatible with purified and crude sample matrices, providing versatility across various gene therapy workflows.
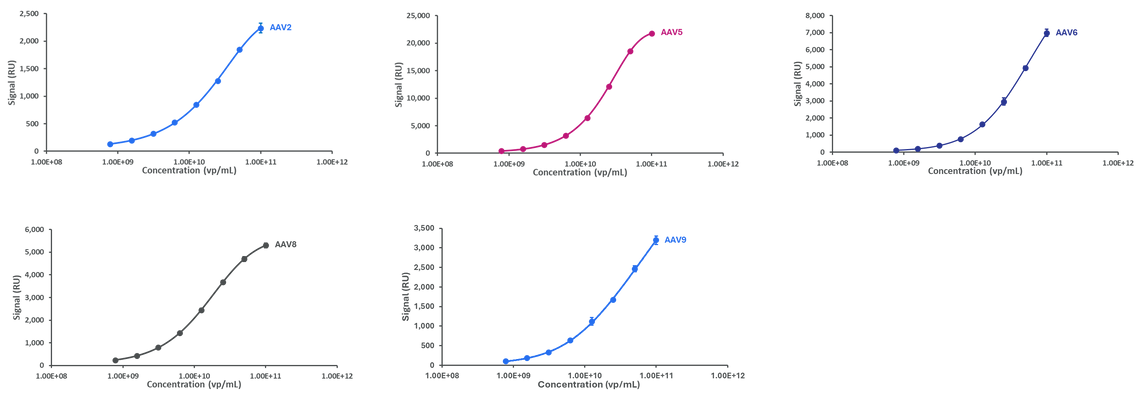
Representative standard curves generated using the AAVX Total Capsid Quantification Kit (AAV2, AAV5, AAV6, AAV8) and the AAV9 Total Capsid Quantification Kit. Image Credit: Abselion
Where Amperia™ supports AAV workflows
Amperia™ enables a variety of AAV workflows, including quick and reproducible quantification across sample types and development phases.
Source: Abselion
| Use Case |
Typical Sample Type |
Value |
| Vector production monitoring |
Crude harvest, clarified lysate |
Track titer during upstream process steps |
| Process optimization |
Samples from variable conditions |
Evaluate yield under different parameters |
| Serotype comparison |
Purified or in-process material |
Assess capsid concentration across variants |
| Method development |
Crude/purified samples |
Streamline assay setup and validation |
Summary
Amperia offers a scalable and streamlined solution for AAV quantification, with sensor-based detection, guided procedures, and broad serotype coverage.
The assay, which uses Thermo Fisher's CaptureSelect™ antibodies, provides consistent results for various sample types. It provides a realistic alternative to traditional procedures when speed, simplicity, and reproducibility are most important.
About Abselion
Abselion started in 2018, at that time under the name HexagonFab, in a small corner of a laboratory at the University of Cambridge.
We set out with the humble goal to make protein research simpler. Scientists should be able to pursue their passion for discovery and innovation, rather than spend their valuable time with tedious, manual tasks. With RED we had access to the ideal technology to create this product. A product that is so compact that it could fit on every bench, and so affordable that it is accessible to everyone. Over the years we have designed, built and tested our first product Amperia and we’re proud to introduce it to the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.