Sponsored Content by BioDlinkReviewed by Maria OsipovaJan 9 2026
The antibody-drug conjugate landscape is facing a critical inflection point. As it stands, manufacturing complexity could pose a threat to future innovation, despite the unmatched market demand.
With over 210 ADCs in clinical development, biotechnology companies are facing an increasingly complicated challenge: managing manufacturing yields, conjugation bottlenecks, and regulatory complications with contract development manufacturing organizations (CDMOs), all while competing with pharmaceutical giants with bountiful resources.1
One solution was published in Acta Pharmaceutica Sinica B by Doctor Wei Huang et al., demonstrating that one-step synthesis of glycosite-specific ADCs could be performed in less than one hour, and achieve 95 % conjugation yields.2
This technological advance addresses the most pressing pain points facing ADC developers and their CDMO partners: manufacturing unpredictability and inefficiencies that can hinder clinical timelines; cost structures that necessitate tens of millions of dollars to be spent on specialized facilities; and difficulties in maintaining quality that create regulatory uncertainty.
A one-step glycoengineering strategy eliminates potential heterogeneity, producing clean and well-defined ADCs, which can be confirmed by size-exclusion chromatography, hydrophobic interaction chromatography, and mass spectrometry.
This all-in-one platform aids therapeutic reliability and manufacturing predictability by significantly simplifying conjugation, enhancing stability, and improving uniformity. Such improvements are critical for productive clinical translation.
The manufacturing challenges facing ADC innovation
Antibody-drug conjugates (ADCs) remain one of the most effective therapeutic classes in oncology, as they combine the potency of cytotoxic payloads with the specificity of monoclonal antibodies. As the ADC sector continues to expand (with over 210 global candidates in clinical development), the ability to reliably and efficiently develop and manufacture will be a key competitive factor.
Despite their promise in clinical settings, the development of ADCs continues to be constricted by bottlenecks during the manufacturing process. Legacy methods for conjugation are often labor-intensive, analytically demanding, and multi-step.
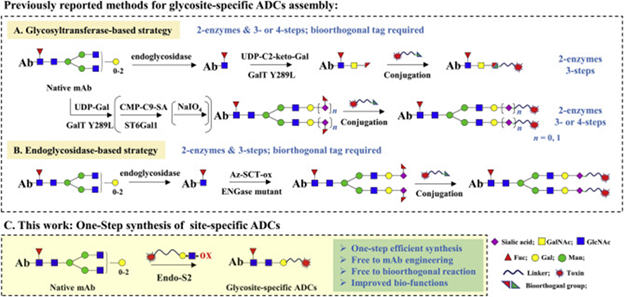
Figure 1. Strategies for synthesis of glycosite-specific ADCs. Image Credit: BioDlink
Such complexities can amplify risk and slow progress for biopharmaceutical and biotechnology companies that operate under financial pressures and time constraints, and for CDMOs that are tasked with delivering high-quality conjugates at scale.
Dr. Wei Huang and team have created a technique that challenges these drawbacks.
The team reprogrammed IgG (Immunoglobulin G, an antibody made by the human body) glycoengineering with LacNAc-based substrates in the presence of wild-type Endo-S2. They demonstrated that native antibodies can be turned into homogeneous glycosite-specific ADCs (gsADCs) in just one step, achieving yields above 95 % in one hour. By using a natural enzyme (Endo-S2) and special sugar blocks (LacNAc) to ‘reprogram’ the sugars on an IgG antibody, it creates a precise landing spot where a cancer-killing drug can be attached, making an ADC much more uniform and reliable.
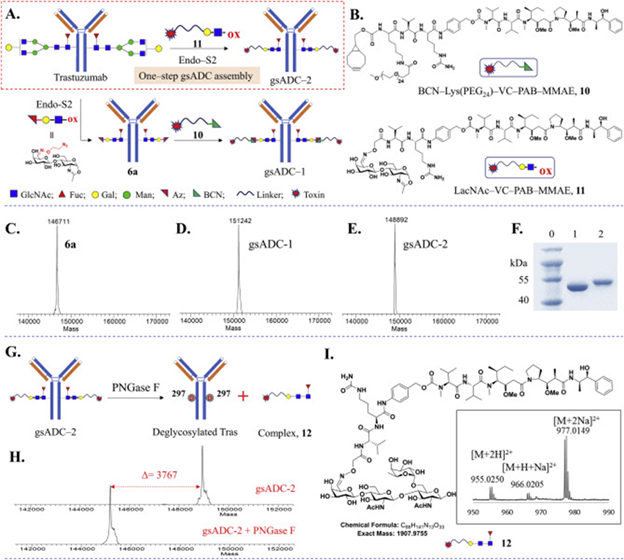
Figure 2. Synthesis of glycosite-specific ADCs using LacNAc-based substrates. (A) General procedure of one-step or two-step synthesis of gsADCs. (B) Small molecules used in gsADCs assembly. (C–E), LC‒MS profiles of Az-LacNAc-Tras 6a (C), gsADC-1 (D) and gsADC-2 (E). (F) SDS-PAGE of one-step synthesis of gsADC-2. Lane 0: protein marker; Lane 1: deglycosylated Tras (1a); Lane 2: gsADC-2. (G) Conjugation-site analysis of gsADC-2 via PNGase F digestion. (H) Antibody molecular weight of gsADC-2 after digestion with PNGase F. (I) HRMS profile of glycan–drug complex released from gsADC-2. Image Credit: BioDlink
Traditional workflows typically require sequential enzymatic or chemical steps to attach linkers, conjugate payloads, and remodel glycans, each of which necessitates intermediate characterization and purification. These incremental processes increase cleanroom occupancy, lengthen production timelines, increase regulatory documentation requirements, as well as the potential for failure points.
In contrast, the one-step LacNAc strategy combines these different steps into a single, streamlined reaction, resulting in reduced scheduling congestion, increased facility throughput, and a more reproducible and reliable platform for client programs. For biotechs, it provides clearer visibility into risk and cost profiles, as well as speedier access to clinical-grade materials.
A significant benefit of the approach is its ability to generate homogeneous ADCs with a defined drug-to-antibody ratio (DAR). Traditional conjugation strategies often produce heterogeneous mixtures, with DAR values ranging from zero to eight, resulting in populations of under-conjugated species with decreased efficacy, as well as over-conjugated molecules with greater toxicity.
This heterogeneity makes quality control and analytical characterization more difficult, since ADC developers must use substantial chromatographic separations and high-resolution mass spectrometry to dissect the distribution of products.
Wei Huang et al. were able to show that their one-step glycoengineering strategy removes this heterogeneity, producing clean and well-defined ADCs that could be confirmed by hydrophobic interaction chromatography, size-exclusion chromatography, and mass spectrometry.
Hydrophobic Interaction Chromatography (HIC) analysis was undertaken, and the data showed that the gsADCs were of high purity and homogeneous (Figure 3B). These novel gsADCs showed solid performance in buffer stability, with only 10.2 % and 14.6 % of aggregations after incubation in 1 × PBS at 60 °C for 36 hours (Figure 3C).
The ability to focus on one uniform molecular species has acute implications for regulatory filings, easing the validation of release specifications and simplifying the definition of critical quality attributes.
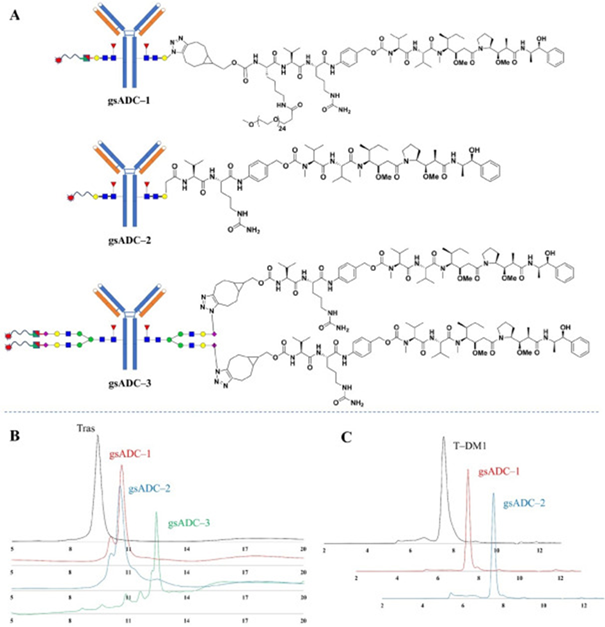
Figure 3. HIC and SEC analysis of gsADCs. (A) Detailed structures of 3 gsADCs. (B) HIC analysis of 3 gsADCs. (C) SEC analysis of gsADC-1 and gsADC-2. Image Credit: BioDlink
Beyond manufacturability, the therapeutic properties of LacNAc-based gsADCs provide solid potential for clinical translation. IC50 is a measurement used to show how much of a drug is required to kill half of the cancer cells in the dish: the lower the value, the stronger the drug.
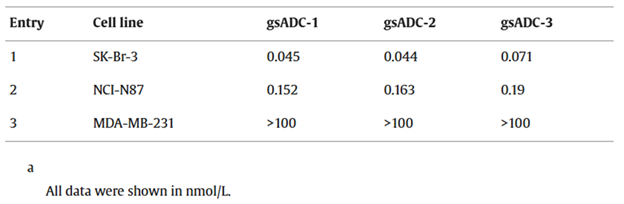
In vitro cytotoxicity assays showed consistent sub-nanomolar potency across multiple HER2-positive cancer cell lines, with IC50 values of 0.045 nM for SK-Br-3 cells, 0.152 nM for NCI-N87 cells (Table 1). Crucially, HER2-negative cell lines exhibited IC50 values higher than 100 nM, indicating a clear therapeutic window and strong selectivity profile.
This pharmacological predictability reduces the uncertainty that can cloud early ADC programs for developers, in which variable DAR distributions can translate into inconsistent potency across batches. By offering reproducible activity aligned with molecular homogeneity, this method creates a more solid foundation for patient stratification and dose selection in clinical development.
Table 1. IC50 values of gsADCs against SK-Br-3, NCI-N87 and MDA-MB-231. Source: BioDlink
In vivo studies further proved the promise of this approach. LacNAc-based gsADCs achieved sustained tumor suppression lasting over 50 days after a single dose in xenograft mouse models, a result that compared favorably to less homogeneous ADC formats, in which tumors generally grew back more quickly.
The LacNAc design is shorter and tucks the toxic drug molecules deeper inside the antibody’s structure, keeping the drug more stable in the bloodstream and helping it reach the tumor more effectively.
Wei Huang et al. compared several versions of ADCs: two made with the new LacNAc method (each with two drug molecules attached per antibody), one made with a different method using cysteine, and another made with a more traditional ‘biantennary sugar’ approach, which carried four drug molecules per antibody. For LacNAc-based gsADC-1 and gsADC-2 (DAR = two), both showed consistent tumor inhibition, exhibiting a better in vivo efficacy than full-glycan gsADC-3 (DAR = four) (Figure 4A and C).
These findings emphasize the translational potential of site-specific conjugation strategies to extend the durability of therapeutic response and improve potency.
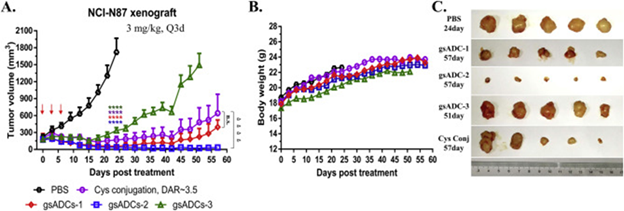
Figure 4. In vivo efficacy assay of 4 ADCs on the NCI-N87 xenograft mice model. (A) Tumor volume curve. Red arrows indicate the time-points of ADC administration. Two-tailed t test was used to assess statistical significance between treatment and control groups. ∗∗∗∗P < 0.0001, comparing all ADCs with vehicle; ▹▹▹▹P < 0.0001, comparing gsADC-2 with Cys-conjugated ADC (n = 5 per group). Data = mean ± SEM. (B) Body weight curve. (C) Tumor images after dissection. Image Credit: BioDlink
The operational benefits of one-step glycoengineering are significant for ADC developers. Since this reaction reaches completion in only one hour, as opposed to traditional conjugation, which can take multiple days, facilities are far more efficient. Shorter processes mean cleanrooms are occupied for less time, freeing up capacity and allowing developers to serve multiple clients.
The standardization of a one-step method simplifies technology transfer, decreasing the time and risks associated with onboarding new programs. With homogeneous products and consistent DAR outcomes, ADC developers can also lessen their dependency on extensive analytical characterization and instead focus on streamlined workflows that verify stability, identity, and purity. Consequently, this shortens project timelines and enables faster delivery of material for preclinical and clinical studies.
By adopting LacNAc-based glycoengineering, ADC developers can differentiate themselves in an increasingly competitive manufacturing landscape. For smaller biotech companies with limited internal CMC resources, being able to rely on CDMO partners with past experience in one-step site-specific conjugation could prove essential for delivering timely clinical entry and avoiding costly program delays. To achieve this, it is beneficial to partner with CDMOs that can deliver high-quality products, accelerate time-to-clinic, and lower overall costs.
The wider implications of this platform also show promise for regulatory management strategies. With homogeneous ADCs, identifying critical quality attributes becomes much easier, enabling developers to set tighter specifications and give regulators clearer evidence of process control. Stability profiles that prove to be resilient under stress strengthen regulatory packages even more, potentially speeding up approval timelines by reducing the need for real-time stability studies, which can take a long time.
Looking ahead, adopting one-step glycoengineering could potentially reshape biotech–CDMO partnership dynamics. Legacy ADC manufacturing has traditionally concentrated capabilities among a small number of global CDMOs with specialized infrastructure.
The risk and complexity of multi-step conjugation limited the availability of feasible partners, often creating capacity shortages and bottlenecks. The reliability and simplicity of the LacNAc-based approach reduce technical barriers, meaning that CDMOs that leverage the LacNAc-based approach become more appealing as potential partners to biotechs.
There are, however, challenges that remain. Scaling this method from laboratory to manufacturing scale requires proof that homogeneity and high yields remain consistent across larger production volumes. In addition, intellectual licensing and property considerations must be navigated to ensure freedom of operation.
Moreover, though regulators are likely to see the enhanced homogeneity and consistency as favorable, ADC developers and CDMOs must establish robust impurity controls and generate comprehensive comparability data, regardless. Addressing these difficulties will be crucial in understanding the full potential of one-step glycoengineering in commercial ADC production.
BioDlink, a leading CDMO, focuses on creating technology to enable process or product development to help biotechs develop their products more efficiently. The company is also committed to making strategic, reliable, and agile alliances that deliver scrupulous fine-tuning to optimize combinations and targets.
Based on the key GL-DisacLink® platform technology developed by Wei Huang and team, BioDlink achieved breakthrough optimizations in the scaling up and streamlining of their processes, resulting in significantly reduced production costs. This is an advanced site-specific conjugation (one-enzyme, one-step) technology that enhances ADC efficacy and stability beyond traditional linker methods.
BioDlink’s proprietary cell line construction platform, BDKcellTM, has a rapid development cycle of 14 weeks. These innovations offer crucial advantages, such as short reaction times, a high level of uniformity, lower production costs, simpler workflows, and reduced off-target effects.
For ADCs, BioDlink can finish the whole process, from DNA synthesis to toxicology study material release, in only seven months, and the full IND application within 11 months. The timelines are six and 10 months for monoclonal antibodies, respectively. It is more important, in the face of global competition, to develop a win-win global ecosystem than to go for the lowest price. Driven by customer needs, the company strives to balance cost, speed, and quality to maximize value.
To conclude, the research conducted by Wei Huang et al. shows that one-step LacNAc-based glycoengineering is not just a laboratory innovation, but rather it represents a crucial pathway for the manufacturing and development of ADCs. This approach explicitly addresses the most important obstructions in ADC development by conflating fast and high-yield conjugation, product homogeneity, better stability, and consistent pharmacological profiles.
About BioDlink Biopharm Co., Ltd.
BioDlink (1875.HK) is a leading global CDMO, specializing in bioconjugates and biologics (ADCs/XDCs). Headquartered in Suzhou with centers in Shanghai and Beijing, the company provides fully integrated, end-to-end services spanning from early research and development through to commercial manufacturing.
With its one-base integrated platform and proprietary technologies, such as GL-DisacLink® for site-specific conjugation and BDKcell® for rapid cell line development, BioDlink helps partners improve efficiency, reduce costs, and accelerate development.
The company operates four commercial manufacturing lines with large-scale sterile fill-finish capabilities, backed by a global GMP-aligned quality system that has earned PMDA accreditation in Japan and supported product approvals across China, Nigeria, Indonesia, Pakistan, Nigeria, Bolivia, and Colombia.
Guided by the philosophy of ‘Quality First, Innovation Driven, Success Together’, BioDlink is committed to advancing global access to next-generation biologics and building trusted partnerships globally.
References:
- Colombo, R., et al. (2024). The Journey of Antibody–Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discovery, [online] pp.OF1–OF20. DOI: 10.1158/2159-8290.cd-24-0708. https://aacrjournals.org/cancerdiscovery/article/14/11/2089/749211/The-Journey-of-Antibody-Drug-Conjugates-Lessons.
- Shi, W., et al. (2022). One-step synthesis of site-specific antibody–drug conjugates by reprograming IgG glycoengineering with LacNAc-based substrates. Acta Pharmaceutica Sinica B, 12(5), pp.2417–2428. DOI: 10.1016/j.apsb.2021.12.013. https://www.sciencedirect.com/science/article/pii/S2211383521004858?via%3Dihub.
About BioDlink
BioDlink (1875.HK) is a leading global CDMO specializing in biologics and bioconjugates (ADCs/XDCs). Headquartered in Suzhou with centers in Shanghai and Beijing, the company provides fully integrated, end-to-end services spanning early R&D through commercial manufacturing.
With its one-base integrated platform and proprietary technologies - such as BDKcell® for rapid cell line development and GL-DisacLink® for site-specific conjugation - BioDlink helps partners accelerate development, improve efficiency, and reduce costs.
The company operates four commercial manufacturing lines with large-scale sterile fill-finish capabilities, backed by a global GMP-aligned quality system that has earned PMDA accreditation in Japan and supported product approvals across China, Indonesia, Nigeria, Pakistan, Colombia, and Bolivia.
Guided by the philosophy of “Quality First, Innovation Driven, Success Together,” BioDlink is committed to advancing global access to next-generation biologics and building trusted partnerships worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.