Cryogenic laboratories carry significant responsibility, including the safe storage of samples for researchers, medical teams, IVF patients, and others. From a financial perspective, these samples can be worth millions, while on a personal level, many are simply irreplaceable.
For any laboratory manager, the thought that an uncontrollable event could destroy those samples is a daunting prospect.
External power outages, equipment malfunctions, alarm failures, or disruptions to liquid nitrogen supply all have the potential to compromise stored samples. In addition to the financial liability associated with sample loss, the reputational impact could be devastating for a laboratory or clinic.
Although complete system failures are relatively rare and therefore often overlooked, they do occur, and when they do, the consequences can be severe.
A perfect storm
There have been several incidents in recent years where a combination of circumstances resulted in the loss of cryopreserved samples.
In late 2023, for example, a freezer malfunction led to the loss of decades of research, primarily focused on leukemia, at the Karolinska Institute in Stockholm. In 2019, a hospital in Los Angeles lost 56 stem cell samples from pediatric cancer patients due to freezer failure.
In 2012, legal action was taken against Edinburgh’s Western General Hospital following damage to stored sperm samples caused by freezer issues. Two separate fertility clinics in Ohio and San Francisco were also sued after catastrophic cryostorage failures.
In another 2012 incident, a hospital in Rome lost 94 embryos when liquid nitrogen levels were no longer sufficient. While the hospital attributed the issue to its liquid nitrogen supplier, an alarm had indicated rising temperatures but went unheard because it was located in the basement, while the laboratory was on the floor above.
Cases such as these highlight the importance of every laboratory having a well-defined emergency plan in place. It is difficult to overstate the importance of such preparation.
An article published by the National Institute of Standards and Technology in the United States provides an example of the contingency planning used in cryogenic storage facilities to manage power outages caused by hurricanes.
Every laboratory is different, with its own variables and risks. Preparing for all potential scenarios requires an assessment tailored to the specific facility. The challenge is knowing where to begin.
Fail to plan, plan to fail
This long-standing principle is especially relevant in cryogenic storage. Preparing for worst-case scenarios requires advance planning for failures that could occur at any time. What happens if power is lost? What if a freezer fails? What if there is an issue with the liquid nitrogen line or a disruption somewhere along the LN2 supply chain?
Adopting a “what if” approach is the foundation of effective emergency planning. Reviewing each potential issue helps identify vulnerabilities, allowing safeguards to be implemented that protect storage integrity, reduce risk, and ensure regulatory compliance.
The Air Products Biomedical team regularly advises customers on potential failure points and how to strengthen cryogenic storage systems. Two of the most common vulnerabilities identified involve power outages and equipment failure.
A range of solutions addresses these risks. Hospitals and universities may already have uninterruptible power supplies or emergency generators in place, and some liquid nitrogen freezers come equipped with battery backup systems.
To address equipment failure, some organizations maintain backup freezers on standby, although for many laboratories, this approach can be inefficient or cost-prohibitive.
Time is your worst enemy
In the event of a failure, effective mitigation depends largely on one factor: time. The more time available before samples reach critical temperatures, the greater the likelihood that they can be preserved without damage.
For this reason, Air Products emphasizes solutions that maximize response time, such as backup power systems, alarm functionality, and remote monitoring that provides immediate notification of failures.
This is also why high-efficiency liquid nitrogen freezers, including those in the MVE range, are often recommended over ultra-low temperature freezers. While ULT freezers typically offer a hold time of up to 96 hours, High Efficiency models can provide hold times ranging from 13 to 27 days, depending on the model. This significantly increases safety margins and supports uninterrupted preservation.
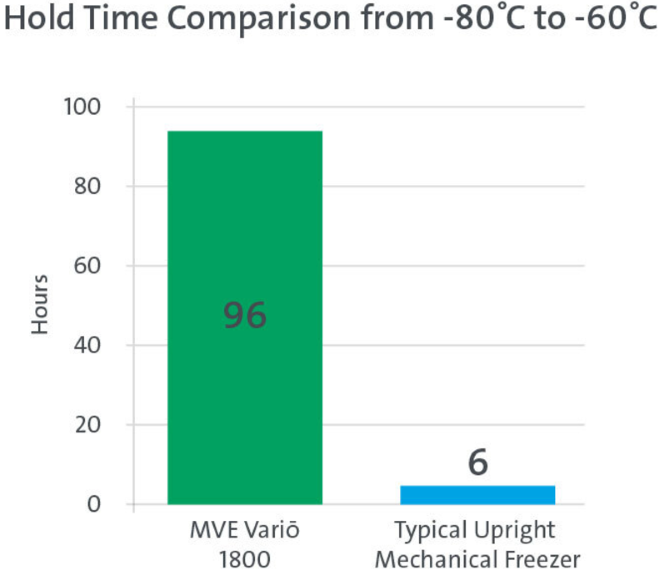
Image Credit: Air Products PLC
In summary
While it is impossible to anticipate every scenario that could disrupt cryogenic storage, the importance of preparation cannot be overstated. Every laboratory should implement a basic checklist to reduce the risk of serious incidents:
- Comprehensive risk assessment: Identifying potential risks enables the implementation of preventive measures.
- Emergency planning: Clear procedures ensure all personnel understand how to respond in an emergency.
- Thorough training: Well-trained staff are better prepared to respond to emergencies and avoid hazards.
- Regular maintenance and safety checks: Establish a strict maintenance schedule to reduce the likelihood of equipment failure.
- Alarms and sensors: Ensure alarms are installed for fire, liquid nitrogen leaks, temperature deviations, and freezer failure, and verify regularly that they function correctly.
This checklist serves as a starting point. Many other issues can arise in cryogenic storage facilities, and it is always better to be overly prepared.
The simple solution
Every storage facility is unique, and many laboratory managers lack the time or specialized expertise required to conduct thorough risk assessments and implement appropriate safeguards.
Working with an experienced and trusted partner can therefore be invaluable in helping ensure that a laboratory is resilient enough to avoid potentially catastrophic events. The Air Products Biomedical team is available as a long-term strategic partner, offering expert guidance based on existing infrastructure, operational needs, location, and budget.
For organizations planning upgrades or new facilities, Air Products can support decision-making that promotes long-term sample security, scalability, and sustainability.
Guidance is also available for integrating new technologies into existing systems, balancing cost and risk for backup solutions, and establishing effective emergency protocols and training programs.
These services are designed to help laboratories define priorities, strengthen operational resilience, and provide lasting peace of mind.
Acknowledgments
This article was produced using materials originally authored by Keith Lewis at Air Products.
About Air Products PLC
Air Products touch the lives of consumers around the globe in positive ways every day. With approximately 16,000 employees and operations in 50 countries, we serve customers across a wide range of industries from food and beverage to medical, energy, and transportation. We supply a unique portfolio of atmospheric and process gases, equipment, and services.
Founded in 1940, Air Products has built a reputation for its innovative culture, operational excellence, and commitment to safety and the environment. Our passionate, talented, and committed employees from a diversity of backgrounds are driven by Air Products’ higher purpose to create innovative solutions that benefit the environment, enhance sustainability and address the challenges facing customers, communities, and the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.