INNOVANCE Antithrombin assay is a cutting-edge, ready-to-use chromogenic reagent for the automated determination of antithrombin (AT) activity.
It is highly accurate even at low activity levels and highly sensitive to diagnosing hereditary and acquired antithrombin abnormalities. The INNOVANCE antithrombin assay was described for use in patients treated with QFITLIA (fitusiran), which supports the AT-based dosage schedule.
Benefits
Siemens Healthineers’ INNOVANCE Antithrombin assay is a liquid, ready-to-use reagent automated for use with different hemostasis systems. The exact combination of human factor Xa, standardized system parameters, and a heparin concentration of 1500 IU/L results in a robust and sensitive assessment of AT activity.
Diagnostic consistency
- Good technique correlation allows for a more consistent interpretation of results
- High accuracy in the low range reduces the need for repeated testing, saving time and reagents
- Excellent sensitivity for detecting antithrombin mutations
Excellent precision
- Available for any-sized lab on the CN-3000/6000,* CS-2500, CS-5100, CA-600, and BCS XP Systems from Siemens Healthineer
- In the clinical decision range of 15 to 35% antithrombin activity, exceptional accuracy is necessary for accurate dosage monitoring of QFITLIA (fitusiran). When analyzing plasma pools, the INNOVANCE Antithrombin assay has shown accurate findings within the decision range with a CV of 5.5% or less
Efficient testing
- Convenient onboard stability allows for effective reagent control
- It uses a multi-analyte calibrator and controls, reduces waste, and offers cost and labor savings. It is available in several ready-to-use kit sizes
Technical details
Technical Specifications. Source: Siemens Healthineers
| . |
. |
| 14 µL |
Required sample volume, depending on system application |
| 120 hours |
Onboard stability on CS-5100 System† |
| 100/130/400 tests per kit |
3 kit configurations available to suit different volume needs |
| 6–150% of norm |
Measuring range on CS-5100 System |
| 3.8% |
Within-device/lab CV (%) with a plasma pool at 15.1% of norm (CS-5100 System) |
| 4 weeks |
Stability once opened at 2–8 °C |
99% AT type II HBS
deficiencies correctly identified |
Testing 643 patients with >10 different mutations |
98% AT type I/II
deficiencies correctly identified |
Testing 954 patients with >45 different mutations
Assay performance can vary from country to country as well as with the system application of the respective assay. The values listed above are provided as examples only. |
Evidence
Reliable performance across mutations and use for patients under QFITLIA treatment
Proven sensitivity
The INNOVANCE Antithrombin assay outperformed existing FXa-based AT assays in identifying 99% of patient samples in trials analyzing 643 patients with type I/II variants, including over 10 distinct heparin-binding-site (AT II-HBS) mutations.
Among them were AT Padua I (Rouen I) and AT Budapest III, which are quite prevalent in Europe. Overall, the INNOVANCE Antithrombin test detected 98% of patient samples in trials with AT type I/II mutations, which is on par with or better than existing AT assays.
Efficient therapy management
The INNOVANCE Antithrombin assay was chosen for clinical studies with the QFITLIA treatment in patients with hemophilia A or B, with or without inhibitors, due to its remarkable capacity to measure within the therapeutic range, when others could not.
This test was cleared as a companion diagnostic for QFITLIA (fitusiran) in the United States.§
Its therapeutic value is acknowledged globally. The assay uses consistent reagents worldwide, albeit the intended usage may differ by location.
Proven precision
The underlying study examined the accuracy and variability of eight antithrombin activity assays used by 48 laboratories in 16 different countries, with a focus on plasma samples that were around or below 30% of the norm.
The authors discovered a greater variety of findings in this range, which is particularly significant for the antithrombin-based dosage regimen with fitusiran. A number of tests showed variability up to 57.5% CV with decreasing AT activity levels; however, the INNOVANCE Antithrombin assay maintained its accuracy.
Clinical use
INNOVANCE Antithrombin assay can be used for the following clinical applications:
- Identify causes of thrombosis also in the presence of pregnancy complications: Detect antithrombin deficiency to help guide optimal anticoagulation methods and make outcome-driven therapy decisions
- Ensure effective heparin therapy: Monitor AT activity levels to determine the effectiveness of heparin-based anticoagulation.
- Support treatment in complex conditions: Help manage patients with DIC, sepsis, or liver cirrhosis by assessing antithrombin levels and tailoring therapy
- NEW! Determine AT-levels in hemophilia A or B patients under QFITLIA therapy: INNOVANCE Antithrombin assay was found to offer excellent support for AT-based QFITLIA (fitusiran) therapy, even in patients who had factor VIII or IX inhibitors. The assay's high accuracy in low activity ranges was regarded as particularly useful for monitoring antithrombin levels, which are crucial to this treatment.
Flexible, high-precision testing to match your lab’s workflow
The INNOVANCE Antithrombin assay provides automated, WHO-standard measurement of FXa-based antithrombin activity. Flexible, high-precision testing with three kit sizes—small, medium, and large—tailored to the lab’s workflow and daily test volume, reducing waste and increasing efficiency.
The test is calibrated with standard human plasma. Control Plasma N and Control Plasma P are allocated values to provide quality control.
Image Credit: Siemens Healthineers
Buffer
Image Credit: Siemens Healthineers
A ready-to-use liquid buffer with preservatives.
Substrate
Image Credit: Siemens Healthineers
A ready-to-use liquid with factor Xa substrate and preservatives in a buffered solution.
Reagent
Image Credit: Siemens Healthineers
Heparin, hirudin, human factor Xa, stabilizers, and preservatives are all present in this ready-to-use liquid.
Use assays from Siemens Healthineers along the official ISTH testing workflow
The ISTH guideline on AT testing proposes a process for antithrombin testing. A step-by-step strategy is advised for classifying antithrombin (AT) deficiency, which includes two types of activity tests and an AT antigen test.
Labs frequently don’t know if a patient is taking anticoagulants, making it difficult to select the appropriate AT test. One alternative is to utilize factor Xa and thrombin-based activity assays in a staggered manner as needed.
The Atellica Data Manager may be configured to handle this technique, including computing the activity-to-antigen ratio.
Note: The overview below does not highlight clinical considerations concerning potential causes of acquired AT deficiency or the impact of a full-dose heparin therapy.
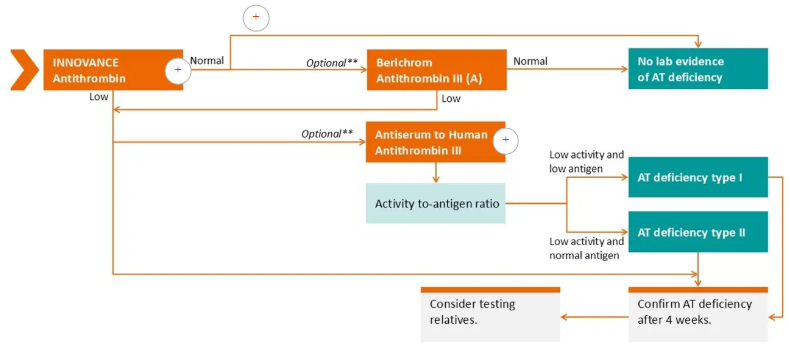
Image Credit: Siemens Healthineers
The INNOVANCE Antithrombin assay can be used as the initial step in improving reagent management and diagnostic accuracy and sensitivity.
The Berichrom Antithrombin III (A) assay can be used as a second step to identify any interference of anti-Xa-based anticoagulants that might be producing the normal result with the INNOVANCE Antithrombin assay if the results of the INNOVANCE Antithrombin assay fall within the normal range.
There has been evidence of an overestimation of AT levels with factor IIa-based AT activity assays in patients receiving factor IIa-based anticoagulant medication, as well as a link between rising rivaroxaban or apixaban concentrations and rising AT findings with factor Xa-based AT activity assays.
Nevertheless, samples from patients treated with anticoagulants that block anti-Xa or anti-IIa and had specific type II mutations still produced abnormal findings from the INNOVANCE Antithrombin and Berichrom Antithrombin III (A) assays. Furthermore, there was a strong technique correlation between the two assays.
AT antigen levels can be determined using the Antiserum to Human Antithrombin III assay. The activity-to-antigen ratio makes it possible to identify AT deficiencies that call for customized care. The testing plan outlined here, including the activity-to-antigen ratio calculation, may be programmed into Atellica Data Manager.
Classifying antithrombin deficiencies
AT defects are classified as type I and type II. While quantitative type I deficit can be acquired or inherited, qualitative type II deficiency is hereditary and is distinguished by an abnormal AT molecule. Type II faults are further classified into three subgroups based on the location of the mutations.
The precise categorization of AT deficiency promotes the appropriate treatment. While heterozygous HBS mutations, depending on the specific mutation, may not always be associated with an elevated thrombotic risk, they might induce pregnancy difficulties. Distinguishing between AT deficiency types is important in determining the appropriate therapy: anticoagulation or AT replacement.
Explore the antithrombin testing portfolio
Expand precision medicine through improved diagnostic accuracy in antithrombin testing
Siemens Healthineers provides antithrombin tests that allow users to diagnose with certainty and efficiency. Choose from functional activity assays targeting factor Xa or IIa, as well as an antigen assay for exact quantification—all of which are tailored for automated processes and consistent findings.
The INNOVANCE Antithrombin assay employs human factor Xa, whereas the Berichrom Antithrombin III (A) assay uses bovine thrombin. They assess AT activity and can identify qualitative and quantitative shortcomings. The third AT assay from Siemens Healthineers, the Antiserum to Human Antithrombin III assay, detects quantitative defects, i.e. AT antigen.
INNOVANCE Antithrombin assay
The INNOVANCE Antithrombin assay is a liquid, automated, chromogenic factor Xa-based activity assay that quantifies antithrombin function using human factor Xa. Siemens Healthineers offers a variety of hemostasis systems.
Berichrom Antithrombin III (A) assay
The Berichrom Antithrombin III (A) assay is an automated, chromogenic thrombin-based activity assay that quantifies antithrombin function using bovine thrombin. Siemens Healthineers offers a variety of hemostasis systems.
Antiserum to Human Antithrombin III assay
The Antiserum to Human Antithrombin III assay is a liquid, automated assay to quantify antithrombin antigen levels. It is available on Atellica NEPH 630** and BN II Systems from Siemens Healthineers.
*The products/features mentioned herein are not commercially available in all countries. Their future availability cannot be guaranteed. Not available for sale in the U.S.
†Using reagent caps.
§As of June 2025.
**Not available for sale in the U.S.