2. What should I know before I use Enspryng?
Do not use if you have ever had an allergic reaction to Enspryng or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section
2. What should I know before I use Enspryng? in the full CMI.
3. What if I am taking other medicines?
4. How do I use Enspryng?
Enspryng is given by injection under the skin (subcutaneously) using a single-dose
pre-filled syringe.
Each injection contains 120 mg of satralizumab. Inject the entire content of the syringe
each time.
The first injection will be given under the supervision of your doctor or nurse.
Follow the instructions provided by your doctor and use Enspryng once every four weeks
for as long as your doctor tells you to. Do not suddenly stop using Enspryng without
asking your doctor first.
5. What should I know while using Enspryng?
|
Things you should do
|
Tell any doctor, dentist, pharmacist or surgeon and anaesthetist you visit that you
are using Enspryng, if you are about to be started on any new medicine or if you are
having surgery.
Tell your doctor about your Enspryng treatment if you are having any blood tests.
Check with your doctor if you are pregnant or intend to become pregnant or if you
are breastfeeding or intend to breastfeed.
Call your doctor straight away if you develop a serious infection while using Enspryng.
|
|
Things you should not do
|
Do not stop using this medicine suddenly or lower the dosage without checking with
your doctor first.
|
|
Driving or using machines
|
Be careful before you drive or use any machines or tools until you know how Enspryng
affects you.
|
|
Looking after your medicine
|
Refrigerate, do not freeze. Store between 2°C and 8°C. Do not shake it. Keep your
Enspryng pre-filled syringe in the carton until it is time to use it to protect it
from light.
If stored at room temperature, the total time out of refrigeration should not be longer
than 8 days at a temperature that does not exceed 30°C.
|
6. Are there any side effects?
Very common side effects are headache, local reaction at injection site (hot flushes,
skin reddening, itching, rash and pain), joint pain and low fibrinogen levels in the
blood (a protein involved in blood clotting). For more information, including what
to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
This medicine is subject to additional monitoring. This will allow quick identification
of new safety information. You can help by reporting any side effects you may get.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems .
Active ingredient(s):
satralizumab
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using Enspryng. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using Enspryng.
Where to find information in this leaflet:
1. Why am I using Enspryng?
Enspryng contains the active ingredient satralizumab. Enspryng is a monoclonal antibody.
Enspryng is used to treat neuromyelitis optica spectrum disorders (NMOSD) in adult
patients, who test positive for an antibody called AQP4-IgG (aquaporin-4 immunoglobulin
G), also known as NMO-IgG (neuromyelitis optica immunoglobulin G).
NMOSD is an autoimmune disease of the central nervous system. This is a condition
where the immune system attacks the central nervous system. NMOSD mainly affects the
optic nerves (nerves found in your eye) and spinal cord. The damage to the optic nerves
causes inflammation - leading to pain and loss of sight. The damage to the spinal
cord causes weakness or loss of movement in the legs or arms, loss of feeling, and
problems with bladder and bowel function.
In a ‘relapse’, or an ‘attack’ of NMOSD, there is inflammation in the nervous system.
This inflammation causes people to have new symptoms, or have symptoms that they have
had before.
Enspryng works by blocking the action of a protein called ‘interleukin-6’ (IL-6).
This protein is involved in inflammation in the body and the levels of this protein
are high in NMOSD. Enspryng may reduce the risk of a relapse or attack of NMOSD.
2. What should I know before I use Enspryng?
Warnings
Do not use Enspryng if:
you notice that it is cloudy, discoloured or contains visible particles.
you are allergic to satralizumab, or any of the ingredients listed at the end of this
leaflet.
you have had an allergic reaction to any other medicine which contains proteins that
are of hamster origin.
Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin
Check with your doctor if you:
have any other medical conditions.
take any medicines for any other condition.
you have allergies to any other medicines, foods, preservatives or dyes.
think you have any signs of infection before, during or after treatment. Signs of
infection may include: fever or chills, cough that does not go away, sore throat,
herpes (such as cold sore, shingles or genital sores), skin redness, swelling, tenderness
or pain, feeling or being sick, diarrhoea or belly pain. If you have an infection,
you will need to delay Enspryng until the infection is controlled.
have recently had been given any vaccines or might be given vaccines in the near future.
You cannot have a ‘live’ or ‘live attenuated’ vaccines while you are being treated
with Enspryng (for example BCG for tuberculosis or vaccines against yellow fever).
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy
Check with your doctor if you are pregnant or intend to become pregnant.
Women able to become pregnant must use effective contraception during treatment with
Enspryng and for at least three months after their last injection of Enspryng.
However, if there is a need to use Enspryng when you are pregnant, your doctor will
discuss the risks and benefits to you and your unborn baby.
Breastfeeding
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Your doctor may advise you to stop breastfeeding during treatment with Enspryng. It
is not known if Enspryng passes into breast milk.
Use in children and adolescents
It is not known whether Ocrevus is safe or effective in children and adolescents under
18 years of age. The safety of Enspryng has been studied in a limited number of paediatric
patients’ above 12 years of age. Safety results were consistent with those in adults.
Use in elderly
It is not known whether Ocrevus is safe or effective in elderly patients over 74 years
of age, because it has not yet been studied in this age group.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect Enspryng.
Your doctor may need to change the dose of some medicines, such as warfarin, carbamazepine,
phenytoin and theophylline.
You cannot have a ‘live’ or ‘live attenuated’ vaccines while you are being treated
with Enspryng
4. How do I use Enspryng?
Follow all instructions given to you by your doctor or pharmacist carefully. They
may differ from the information contained in this leaflet. Use Enspryng exactly as
your doctor has prescribed.
Treatment with Enspryng should be started under the supervision of a specialist doctor
experienced in the treatment of NMOSD.
How much and how to use
Enspryng is given by injection under the skin (subcutaneously) using a single-dose
pre-filled syringe. Each injection contains 120 mg of satralizumab. Inject the entire
content of the syringe each time.
The first injection will be given under the supervision of your doctor or nurse.
Once you have been trained, your doctor may decide that you or your caregiver can
inject Enspryng.
The first three injections (loading dose) are given once every two weeks.
After this, the injection (maintenance dose) is given once every four weeks.
Follow the instructions provided and use Enspryng once every four weeks for as long
as your doctor tells you to. Do not suddenly stop using Enspryng without asking your
doctor first.
After removing the cap, the injection must be started within 5 minutes to prevent
the medicine from drying out and blocking the needle. If the pre-filled syringe is
not used within 5 minutes of cap removal, you must dispose of it in a sharps container
and use a new pre-filled syringe.
Directions for self-injection
Your doctor should show you how to prepare and inject properly before you or your
caregiver inject for the first time. Ask them any questions you or your caregiver
may have.
Do not attempt to administer an injection until you or your caregiver understand how
to self-inject Enspryng.
It is important to remain under your doctor's care while using Enspryng. It is recommended
you have someone else present when you self-inject Enspryng in case you experience
any symptoms of a serious allergic reaction described under Section
2. What should I know before I use Enspryng?
The syringe is for single use only and should be safely discarded after use.
How to inject Enspryng
The syringe components:
Before use
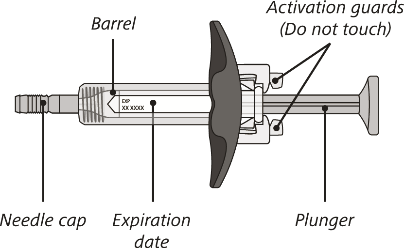
After use
The syringe has a needle-shield that automatically covers the needle when the injection
is complete.
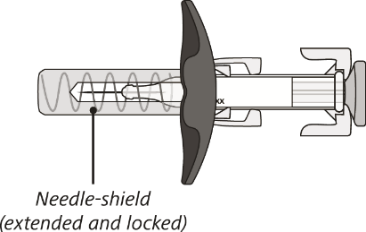
Do not shake the syringe.
Do not try to open the syringe or take it apart.
Do not re-use the same syringe.
Gather what you will need:
Included in the pack:
1 pre-filled syringe
Not included in the pack:
Alcohol pad
Sterile cotton ball or gauze
Sharps container for safe disposal of the needle cap and used syringe.
STEP 1. Prepare to use Enspryng
Take the carton containing the syringe out of the refrigerator and place it on a clean,
flat work surface.
Check the expiry date on the back of the carton. Do not use if the carton has expired.
Check that the front of the carton is sealed. Do not use if the seal has been broken.
STEP 2. Remove pre-filled syringe from the carton
Open the sealed carton and carefully lift the syringe out of the carton by holding
the barrel.
Do not turn the carton upside down to remove the syringe.
Do not touch the activation guards as this may damage the syringe.
Do not hold the plunger or needle cap.
STEP 3. Visual inspection of the pre-filled syringe
Check the expiration date on the syringe. Do not use the syringe if it has expired.
Check the syringe for any damage. Do not use if it is cracked or broken.
Check that the liquid through the viewing window is clear and colourless to slightly
yellow.
Do not inject the medicine if the liquid is cloudy, discoloured, or has particles
in it.
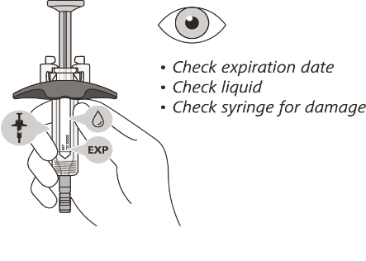
There may be some small air bubbles in the syringe. This is normal and you should
not try to remove them.
STEP 4. Prepare to inject Enspryng
Let your syringe reach room temperature.
Place it on a clean, flat work surface (like a table) for 30 minutes to allow it to
reach room temperature.
It is important to let the syringe gently warm up as injecting cold medicine may feel
uncomfortable and make it harder to push.
Wash your hands with soap and water.
Choose your injection site in either:
the lower part of your stomach (abdomen) or
the front and middle of your thighs.
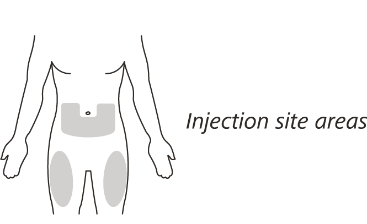
Do not inject into the 5 cm area around your belly button.
Do not inject into moles, scars, bruises, or areas where the skin is tender, red,
hard or broken.
Choose a different injection site for each new injection - choose a different place
to inject which is at least 2.5 cm away from the place where you last injected.
Clean the injection site by wiping it with an alcohol pad and let it air dry.
Do not fan or blow on the area which you have cleaned.
Do not touch the injection site again before you inject Enspryng.
STEP 5. Inject Enspryng
Hold the barrel of the syringe between your thumb and index finger. With your other
hand, pull the needle cap straight off.
You may see a drop of liquid at the end of the needle - this is normal and will not
affect your dose.
Use the syringe within 5 minutes of removing the cap or the needle may clog.
Do not take the needle cap off until you are ready to inject Enspryng.
Do not put the needle cap back on once it has been removed as this may damage the
needle.
Do not touch the needle or let it touch any surfaces after removing the needle cap.
Hold the barrel of the syringe using your thumb and index finger. With your other
hand, pinch the area of skin you have cleaned.
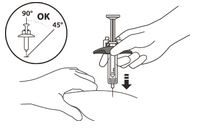
Use a quick, dart-like motion to insert the needle at an angle between 45° to 90°
Do not insert the needle through clothing.
Do not change the angle of the injection.
Do not insert the needle again.
After the needle is inserted, let go of the pinched skin.
Slowly inject all of the medicine by gently pushing the plunger all the way down until
it touches the activation guards.
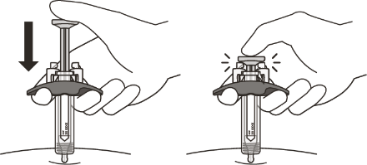
Gently release the plunger and allow the needle to come out of the skin at the same
angle it was inserted.
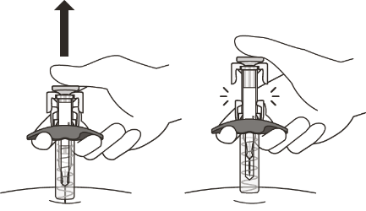
The needle will now be covered by the needle-shield. If the needle is not covered,
carefully place the syringe into a sharps container to avoid injury.
STEP 6. How to take care of the injection site
There may be a little bleeding at the injection site. You can press a cotton ball
or gauze over the injection site but do not rub it. If needed, you may also cover
the area you injected with a small bandage. If the medicine gets into contact with
your skin, wash the area with water.
STEP 7. Disposal of Enspryng
Do not try to re-cap your syringe.
Put your used syringe in a sharps container immediately after use.
Do not throw away (dispose of) the syringe in your household waste and do not recycle
it.
Ask your doctor or pharmacist for information about where you can get a sharps container
or what other types of puncture-resistant containers you can use to safely dispose
of your used syringes and needle caps.
If you forget to use Enspryng
If you forget your scheduled injection, talk to your doctor on how to continue Enspryng
treatment.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you use too much Enspryng
If you think that you have used too much Enspryng, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Enspryng?
Things you should do
Tell your doctor and pharmacist or surgeon and anaesthetist that you are using Enspryng,
if you are about to be started on any new medicine or if you are having surgery.
Tell your doctor about your Enspryng treatment if you are having any blood tests.
It may interfere with the results of some tests.
Tell your doctor develop an infection while using Enspryng. You may need to stop using
Enspryng until the infection is controlled.
Keep all of your doctor's appointments so that your progress can be checked.
Call your doctor straight away if you:
develop a serious infection while using Enspryng. You will need to stop using Enspryng
until the infection is controlled.
have any of the following signs of increased liver enzymes during or after treatment:
yellowing of the skin and the whites of the eyes (jaundice), dark coloured urine,
feeling and being sick. Enspryng can affect your liver function and increase the amount
of some liver enzymes in your blood. Your doctor will do blood tests to check these
amounts and monitor how well your liver is working.
Remind any doctor, dentist or pharmacist you visit that you are using Enspryng.
Things you should not do
Do not stop using this medicine or lower the dosage without checking with your doctor
first.
Do not use Enspryng to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as
you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Enspryng
affects you.
This medicine is not expected to affect your ability to drive a car or operate machinery.
Looking after your medicine
Keep your Enspryng pre-filled syringe in the carton until it is time to use it to
protect it from light.
Refrigerate, do not freeze. Store between 2°C and 8°C. Do not shake it.
If stored at room temperature, the total time out of refrigeration should not be longer
than 8 days at a temperature that does not exceed 30°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it away from moisture, heat or sunlight; for example, do not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the following information and, if you need to, ask your doctor or pharmacist if
you have any further questions about side effects.
The most commonly reported systemic symptoms were diarrhoea and headache.
Less serious side effects
|
Less serious side effects
|
What to do
|
|
Nervous system:
Headache
Migraine
Difficulty in sleeping (insomnia)
Injection-related reactions:
local reaction at injection site (hot flushes, skin reddening, itching, rash and pain)
Joint or muscle:
Joint pain (arthralgia)
Muscle stiffness
Skin:
Rash
Itching
Nose:
Runny or stuffy nose, sneezing
Some side effects can only be found when your doctor does blood tests from time to
time to check your progress (for example, decreased white blood cell counts and increased
bilirubin levels).
Blood:
Low fibrinogen levels in the blood (a protein involved in blood clotting)
Other:
Fluid retention causing swelling in the lower legs or hands (oedema peripheral)
|
Speak to your doctor if you have any of these less serious side effects and they worry
you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|
Injection-related reactions:
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Enspryng contains
|
Active ingredient
(main ingredient)
|
Each pre-filled syringe contains 120 mg of satralizumab
|
|
Other ingredients
(inactive ingredients)
|
histidine
aspartic acid
arginine
poloxamer
water for injections
|
|
Potential allergens
|
None
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes
|
Do not take this medicine if you are allergic to any of these ingredients.
What Enspryng looks like
Enspryng is registered on the ARTG as AUST R 326047.
Each pack of Enspryng contains 1 pre-filled syringe which contains a colourless to
slightly yellow liquid.
Who distributes Enspryng
Roche Products Pty Ltd
ABN 70 000 132 865
Level 8, 30-34 Hickson Road
Sydney NSW 2000
Australia
How to contact us

You can also call us on 1800 233 950.
This leaflet is for people in Australia only. If you are not in Australia, you can
contact Roche/Genentech in your country at
www.medinfo.roche.com .
This leaflet was prepared in July 2023.