What Are Organoids and How Are They Made?
Applications in Disease Modeling and Drug Testing
Comparison with Animal Models
Regulatory Landscape and Industry Adoption
Future Outlook: A Full Replacement or Integration Tool?
Conclusion — A Game Changer in Progress
References
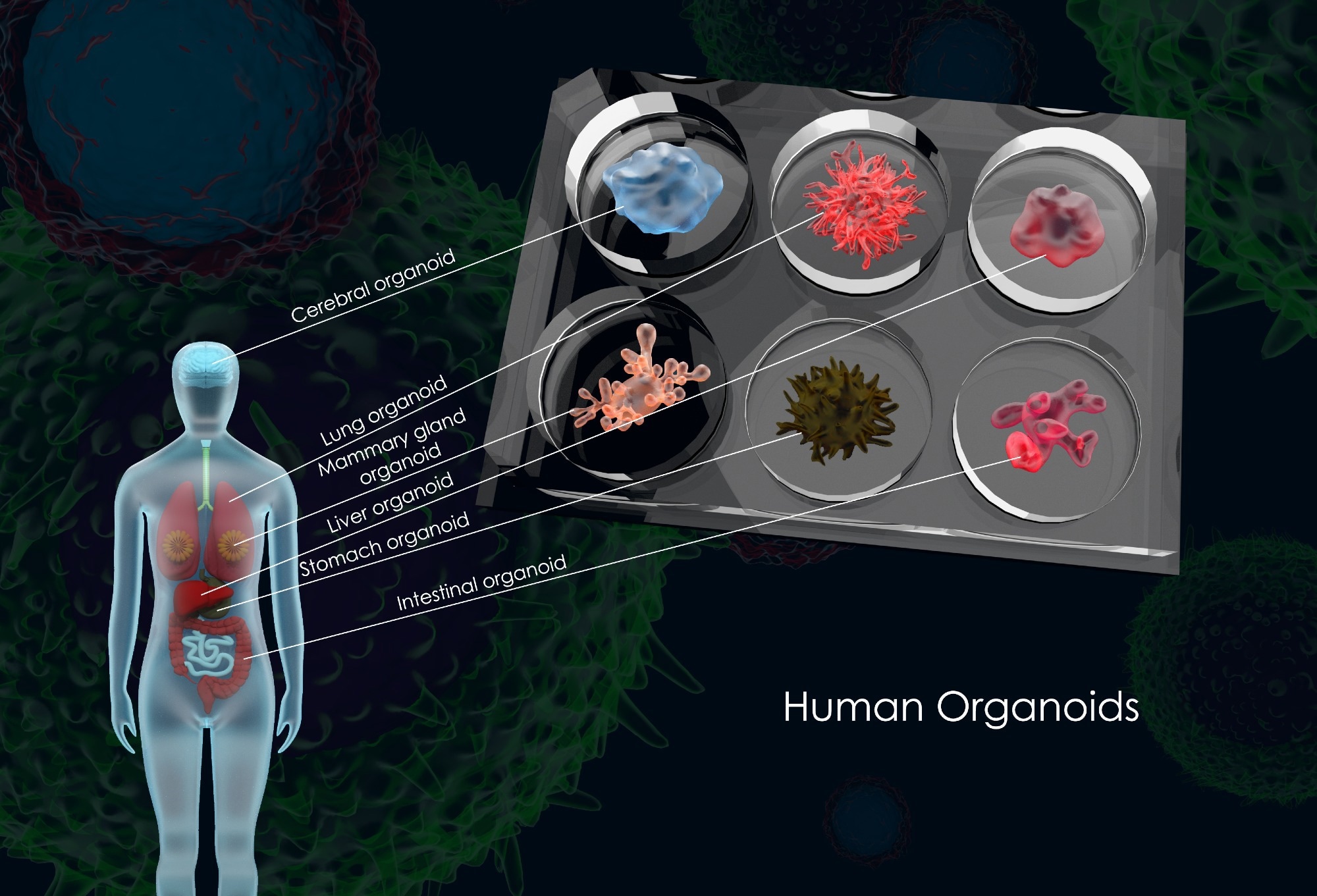 3D illustration of common human organoids, Image Credit: Meletios Verras / Shutterstock
3D illustration of common human organoids, Image Credit: Meletios Verras / Shutterstock
Every year, more than 100 million animals are used globally in biomedical research, yet over 90% of drugs that appear effective in animal trials fail during human clinical testing.1 This staggering disconnect raises a critical question: Are we relying on the wrong models to understand and treat human diseases?
In response, a new frontier in biomedical science has emerged — organoids. Organoids are miniaturized, simplified versions of human organs that are grown in vitro from stem cells. These three-dimensional cellular structures self-organize to mimic the architecture and function of their tissue of origin, offering a promising new avenue for studying human development, disease, and drug responses.2,3
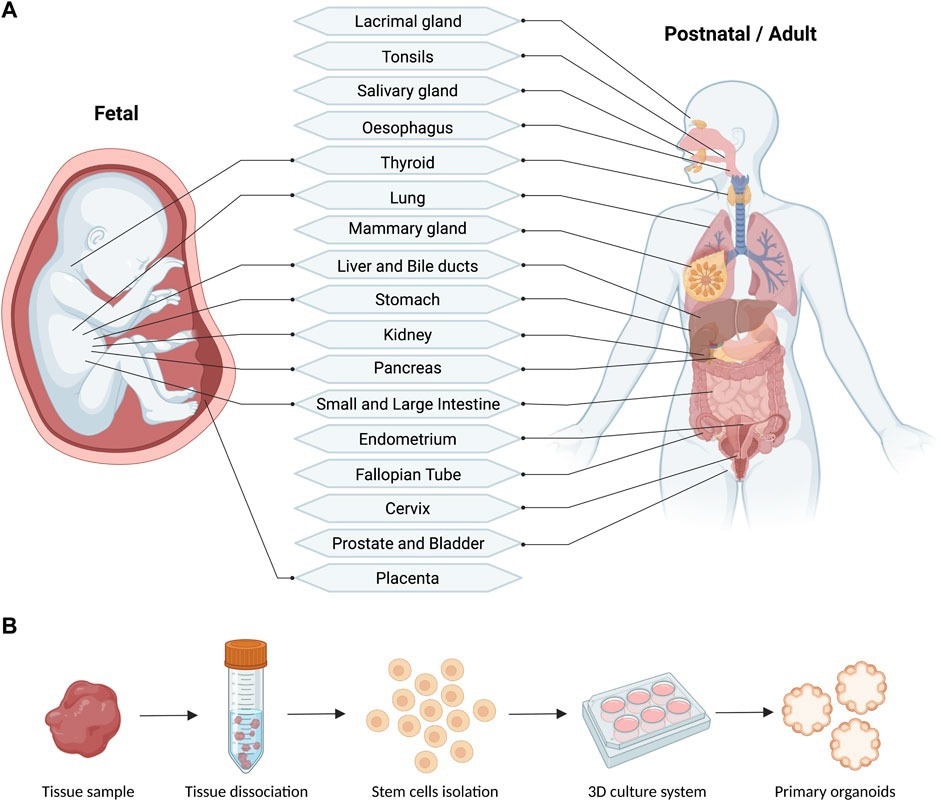
Schematic (A) and workflow (B) of the reported fetal and postnatal/adult tissues used for the derivation of primary human organoids (generated with BioRender).2
Ethical concerns, high attrition rates in drug development, and significant advances in stem cell technology drive this mounting interest in reducing or even replacing animal models. However, the growing popularity of these models in academic, regulatory, and industrial settings also poses the question of whether organoids can fully replicate the complexity of human disease and ultimately replace animal models in biomedical research.
What Are Organoids and How Are They Made?
Organoids are three-dimensional (3D) multicellular structures that are grown in vitro and mimic key functional and structural aspects of real human organs. They are typically derived from pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), or adult stem/progenitor cells isolated from tissue biopsies.2
The process begins by guiding these stem cells through a series of developmental cues using growth factors, extracellular matrix components, and mechanical signals to induce them to self-organize into complex structures resembling the target organ. These cues mirror embryonic development and are tailored to produce specific tissue types. The resulting organoids exhibit cell diversity, polarity, and in some cases, rudimentary organ-level functions.2,4
Numerous types of organoids have been successfully developed since the inception of this technology, including brain organoids with layered neural tissues, liver organoids displaying metabolic and detoxification functions, intestinal organoids with crypt-villus structures, and kidney, pancreatic, and tumor-derived models.3,4
Organoids are distinct from traditional two-dimensional (2D) monolayer cultures, which lack spatial complexity, and from spheroids, which are less organized and do not mimic organ-specific architecture or function. Moreover, their ability to retain patient-specific genetic and epigenetic profiles also makes them valuable tools for personalized medicine. However, the reproducibility and scalability of organoid culture systems remain ongoing technical challenges.4,5
Organoids: mini-tissues in culture
Applications in Disease Modeling and Drug Testing
Organoids have emerged as powerful platforms for modeling human diseases and evaluating therapeutic responses in a physiologically relevant, human-specific context. Their ability to recapitulate the 3D structure, cellular diversity, and gene expression patterns of native tissues makes them especially valuable in studying complex diseases that are difficult to model in animals or 2D cultures.
For example, brain organoids have been instrumental in uncovering how the Zika virus disrupts neurodevelopment, leading to microcephaly. These models showed that the virus preferentially targets neural progenitor cells — a finding that was not observed in studies that used traditional murine models.6
Similarly, gut organoids have transformed cystic fibrosis research by offering personalized platforms for drug testing. Patient-derived intestinal organoids can predict cystic fibrosis transmembrane conductance regulator (CFTR) modulator response, including the response for rare mutations, which has enabled the development of tailored therapeutic strategies.7
In oncology, patient-derived tumor organoids preserve the molecular and histological characteristics of the original tumor, offering a highly personalized platform for drug screening. Liver cancer organoids, for example, have been used to identify subtype-specific vulnerabilities and novel therapeutic targets, such as extracellular signal-regulated kinase (ERK) pathway inhibitors.2–4
Beyond individual case studies, organoids are also increasingly being used in high-throughput drug screening to assess toxicity and efficacy profiles. Their ability to model patient-specific responses allows researchers to stratify treatment regimens, reducing trial-and-error in clinical settings and advancing the goals of precision medicine.2,5
Comparison with Animal Models
Animal models, especially mice, have long been the cornerstone of biomedical research due to their physiological similarities to humans and their manipulability. However, cross-species differences in gene expression, developmental timing, and immune responses have been a limiting factor in the relevance of animal models for human disease.3
Organoids present several advantages over animal models:
- Human specificity: They are derived from human cells, thus avoiding interspecies variability.
- Ethical considerations: The use of organoids reduces the need for animal use in research.
- Faster timelines: Organoids can be cultured and analyzed in weeks, compared to months or years for animal studies.
However, organoids also have certain limitations. They lack the systemic interactions found in whole organisms, such as immune system interplay, hormonal regulation, and blood flow dynamics, which are critical for studying infections and immunotherapies. Most organoids are also limited in size, vascularization, and longevity, which constrains their utility in studying chronic conditions or whole-body pharmacokinetics 3,5
Nevertheless, organoids surpass animal models in capturing species-specific disease processes. For example, mouse models failed to replicate Zika-induced microcephaly unless the virus was injected directly into fetal brain tissue, whereas brain organoids naturally recapitulated the condition due to human-specific cellular responses.3,6
The integration of organoids with in silico models and microfluidic platforms like organs-on-a-chip further enhances their physiological relevance. These hybrid systems can mimic multi-organ interactions and dynamic microenvironments, bridging the gap between in vitro models and in vivo biology.8
Read the latest research news on organoids
Regulatory Landscape and Industry Adoption
With increasing regulatory recognition, organoid models are gaining traction in the pharmaceutical industry. The United States (U.S.) Food and Drug Administration (FDA) Modernization Act 2.0, passed in 2022, reduced mandatory animal testing requirements for preclinical drug development, allowing for validated non-animal alternatives such as organoids to be used for investigating new drug applications.1
Pharmaceutical and biotech companies are now actively integrating organoid models into their research and development strategies. Hubrecht Organoid Technology, a spin-off of the Hubrecht Institute, has partnered with global pharmaceutical companies to provide patient-derived organoids for drug screening and biomarker discovery. Similarly, Emulate Inc. uses organoids in conjunction with organ-on-chip systems to simulate tissue interfaces and physiological responses with high precision.9
These platforms are also attracting significant investment. Venture capital and public-private partnerships have channeled substantial funding into 3D tissue technologies, particularly in oncology, gastrointestinal disease, and neurology. This growing financial commitment underscores industry confidence in organoid systems as scalable, predictive, and ethically advantageous alternatives to animal models.9
However, regulatory agencies still require robust standardization and validation before organoid data can fully replace animal studies. Moreover, issues such as batch-to-batch variability, scalability, and reproducibility remain significant hurdles.9
Future Outlook: A Full Replacement or Integration Tool?
Organoids are poised to become indispensable tools in integrated research strategies. Future improvements, such as vascularization, the incorporation of immune cells, and automation for high-throughput screening, could expand their applicability.5
Furthermore, initiatives such as the Human Cell Atlas are advancing the field through the development of standardized protocols, funding, and cross-disciplinary collaboration.10 As technologies such as gene editing, single-cell sequencing, and bioengineering evolve, organoids will continue to further our understanding of the complexity of human tissues and diseases.
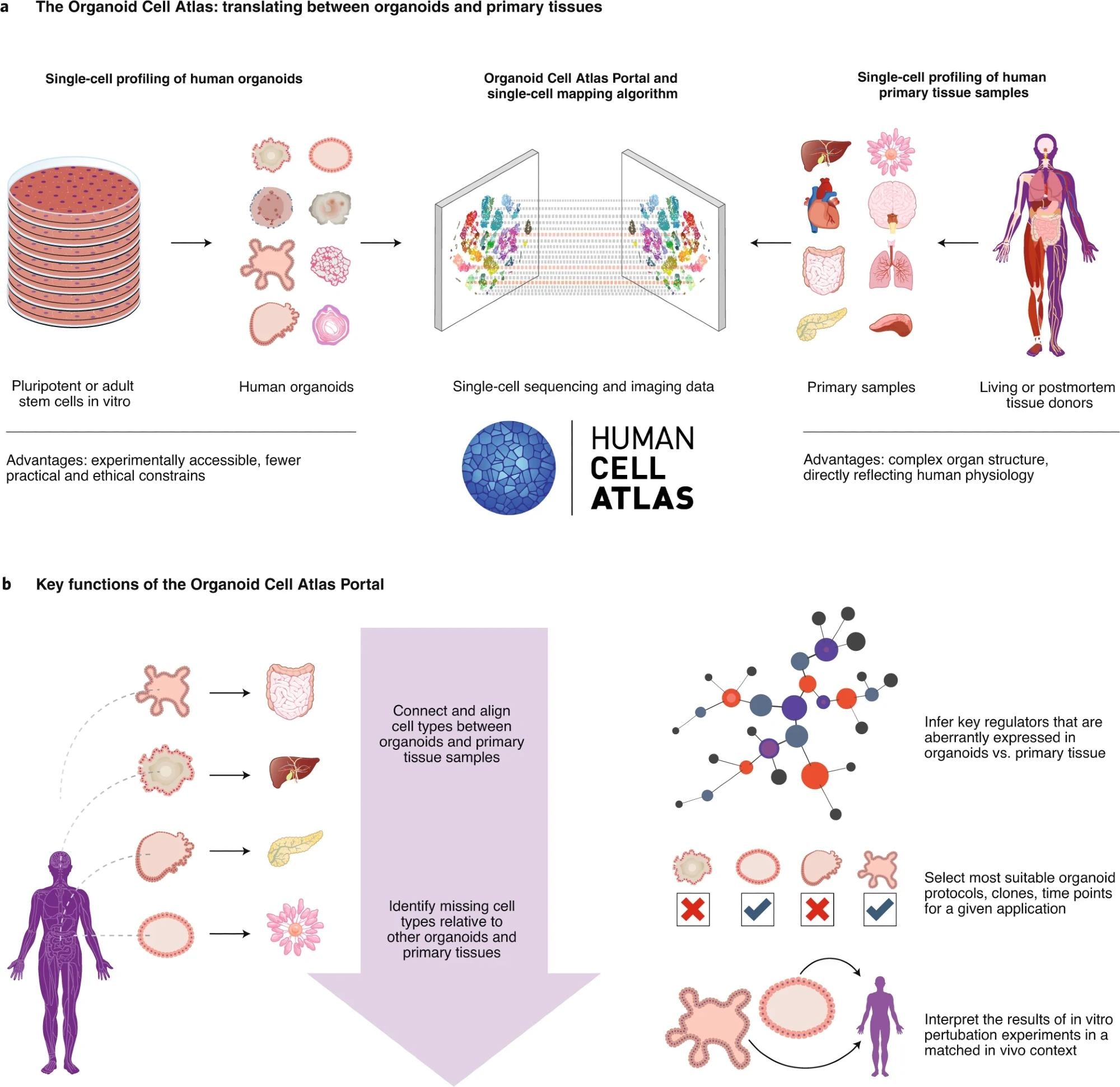 a, Single-cell profiling of human organoids (left) and of human primary tissue samples (right) provides complementary information. Data integration between single-cell profiles from organoids and primary tissues makes it possible to investigate the same cell type in both contexts, allowing each approach to play to its strengths. b, The Organoid Cell Atlas Portal will implement key features for analyzing and interpreting single-cell data from human organoids in the biological context provided by HCA profiles of their in vivo counterparts. 10
a, Single-cell profiling of human organoids (left) and of human primary tissue samples (right) provides complementary information. Data integration between single-cell profiles from organoids and primary tissues makes it possible to investigate the same cell type in both contexts, allowing each approach to play to its strengths. b, The Organoid Cell Atlas Portal will implement key features for analyzing and interpreting single-cell data from human organoids in the biological context provided by HCA profiles of their in vivo counterparts. 10
Conclusion — A Game Changer in Progress
Organoids represent a transformative step in the field of human disease research. Their ability to replicate key features of human tissues offers an opportunity to reduce reliance on animal models, increase experimental relevance to humans, and personalize medicine.
Furthermore, although organoids are not yet ready to entirely replace animal models, their strengths in human-specific biology, drug testing, and disease modeling make them vital to biomedical research. The future of disease modeling lies in integrating organoids, animal studies, and computational models to accelerate the development of safe and effective therapeutics.
References
- Zushin, P. H., Mukherjee, S., & Wu, J. C. (2023). FDA Modernization Act 2.0: transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. The Journal of Clinical Investigation, 133(21), e175824. DOI:10.1172/JCI175824, https://www.jci.org/articles/view/175824
- Calà, G., Sina, B., De Coppi, P., Giobbe, G. G., & Gerli, M. F. M. (2023). Primary human organoid models: Current progress and key milestones. Frontiers in Bioengineering and Biotechnology, 11, 1058970. DOI:10.3389/fbioe.2023.1058970, https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2023.1058970/full
- Corsini, N. S., & Knoblich, J. A. (2022). Human organoids: New strategies and methods for analyzing human development and disease. Cell, 185(15), 2756–2769. DOI:10.1016/j.cell.2022.06.051, https://www.sciencedirect.com/science/article/pii/S0092867422008443
- Lehmann, R., Lee, C. M., Shugart, E. C., Benedetti, M., Charo, R. A., Gartner, Z., Hogan, B., Knoblich, J., Nelson, C. M., & Wilson, K. M. (2019). Human organoids: a new dimension in cell biology. Molecular Biology of the Cell, 30(10), 1129–1137. DOI:10.1091/mbc.E19-03-0135, https://www.molbiolcell.org/doi/10.1091/mbc.E19-03-0135
- Zhu, Z., Cheng, Y., Liu, X., Ding, W., Liu, J., Ling, Z., & Wu, L. (2025). Advances in the Development and Application of Human Organoids: Techniques, Applications, and Future Perspectives. Cell Transplantation, 34, 9636897241303271. DOI:10.1177/09636897241303271, https://journals.sagepub.com/doi/10.1177/09636897241303271
- Qian, X., Nguyen, H. N., Jacob, F., Song, H., & Ming, G. L. (2017). Using brain organoids to understand Zika virus-induced microcephaly. Development (Cambridge, England), 144(6), 952–957. DOI:10.1242/dev.140707, https://journals.biologists.com/dev/article-abstract/144/6/952/48342/Using-brain-organoids-to-understand-Zika-virus
- de Poel, E., Lefferts, J. W., & Beekman, J. M. (2020). Intestinal organoids for Cystic Fibrosis research. Journal of Cystic Fibrosis, 19 Suppl 1, S60–S64. DOI:10.1016/j.jcf.2019.11.002, https://www.sciencedirect.com/science/article/pii/S1569199319309646
- Park, S. E., Georgescu, A., & Huh, D. (2019). Organoids-on-a-chip. Science, 364(6444), 960–965. DOI:10.1126/science.aaw7894
- Zhou, L., Huang, J., Li, C., Gu, Q., Li, G., Li, Z. A., Xu, J., Zhou, J., & Tuan, R. S. (2025). Organoids and organs-on-chips: Recent advances, applications in drug development, and regulatory challenges. Med, 6(4), 100667. DOI:10.1016/j.medj.2025.100667, https://www.sciencedirect.com/science/article/pii/S2666634025000947
- Bock, C., Boutros, M., Camp, J. G., Clarke, L., Clevers, H., Knoblich, J. A., Liberali, P., Regev, A., Rios, A. C., Stegle, O., Stunnenberg, H. G., Teichmann, S. A., Treutlein, B., Vries, R. G. J., & Human Cell Atlas ‘Biological Network’ Organoids (2021). The Organoid Cell Atlas. Nature Biotechnology, 39(1), 13–17. DOI:10.1038/s41587-020-00762-x, https://www.nature.com/articles/s41587-020-00762-x
Last Updated: May 2, 2025