This article is based on a poster originally authored by Tianfu Zhang, Ya Zhao, Na Zhang, Xing Zhang, Haonan Li, Spencer Chiang and Yuehchun Hsieh.
Natural killer (NK) cells are large granular immune cells that play an important role in anti-inflammatory responses and in monitoring tumors.1
When using NK cells in adoptive immunotherapy, acquiring enough high-quality NK cells is a major challenge. The ability of induced pluripotent stem cells (iPSCs) to differentiate into different cell types in vitro offers hope for cell treatments.2
A unique and reliable approach for inducing NK cells from iPSCs (iNK) in a completely feeder-free environment was designed in this study. After 35 days, the cell proportion of CD3- CD56+ iNK Cells reached 96.33 %, and by day 49, the expansion fold had reached 70 K, suggesting the feasibility of large-scale NK cell production for clinical applications.
Differentiation from iPSC to NK cells
A culture system was designed to optimize the differentiation of iPSC to iNK cells. NOTCH ligands DLL4 and VCAM-1 provided a supportive environment for HSPC differentiation into the NK lineage.
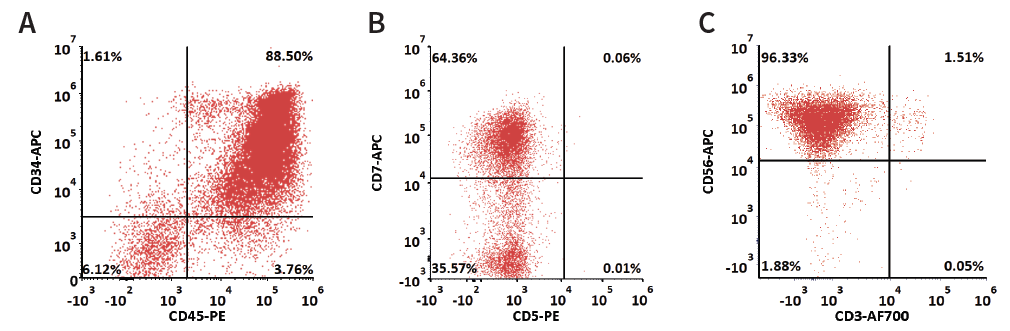
Figure 1. Flow cytometry analysis of cell components throughout the differentiation and expansion process. Cell percentages of (A) 88.5 % of HSPCs by Day 14, (B) 64.32 % of CLPs by Day 21, and (C) 96.33 % of iNKs by Day 35. Image Credit: ACROBiosystems
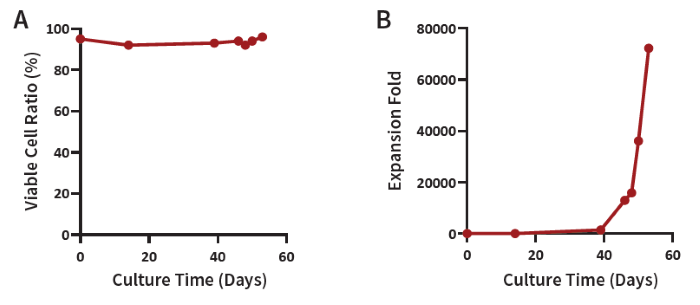
Figure 2. (A) iNK cell viability throughout the culture process and (B) expansion fold. After NK cell maturity, expansion reaches over 70K-fold, with an average survival rate of 95 % throughout the differentiation/expansion process, highlighting its potential as a robust NK cell source in only 49 days. Image Credit: ACROBiosystems
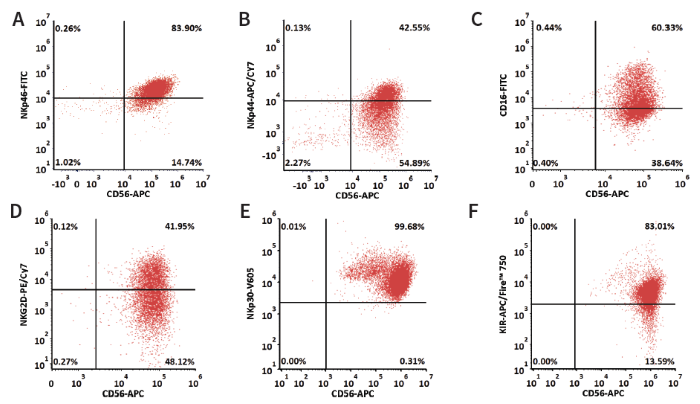
Figure 3. After a 35-day NK cell expansion period, iNKs were analyzed for NK surface markers including (A) NKp46, (B) NKp44, (C) CD16, (D) NKG2D, (E) NKp30, (F) KIR to confirm cell identity and function. High percentages of marker-positive cells reveals the success of NK cell differentiation. Image Credit: ACROBiosystems
iPSC differentiation pathway
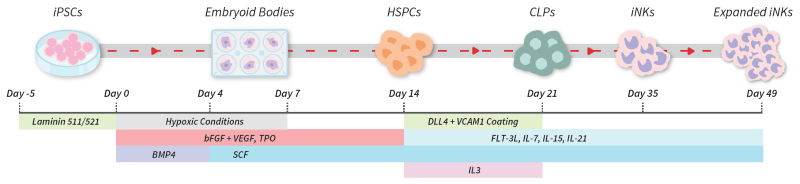
Image Credit: ACROBiosystems
Degranulation marker CD107a & secreted IFN-γ
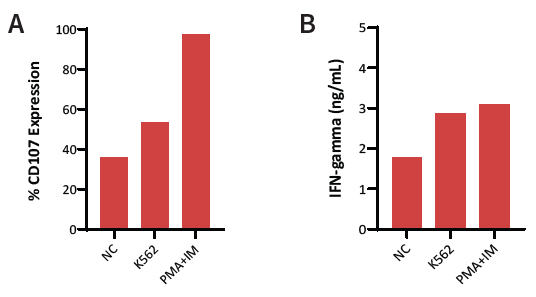
Figure 4. iNK (Day 35) and leukemia (K562) cells were co-cultured at various E:T ratios. (A) CD107a marker revealed middling degranulation of iNKs between negative (NC) and positive (PMA+IM) controls, revealing a 53.5 % expression of CD107a. (B)Secretion of IFN-γ at a high level, mimicking cytokine secretion at levels close to the chemically-induced positive control. Image Credit: ACROBiosystems
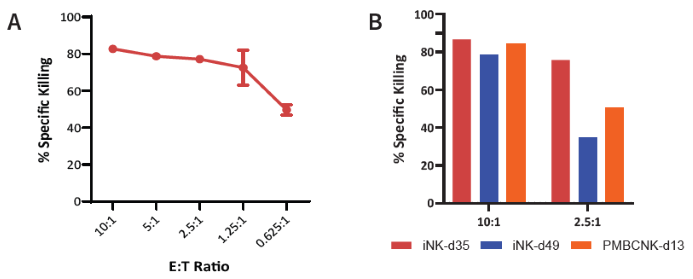
Figure 5. Measurement of remaining target cells was performed after 7-AAD/CFSE staining and analyzed via flow cytometry. (A) iNK (Day 35) cytotoxicity increased with E:T ratios, revealing > 40 % of target cells lysed at the lowest ratio. (B) During a comparison between PBMC-derived NKs vs iNKs from this method, Day 35 iNKs showed the highest cytotoxicity against K562 cells. Image Credit: ACROBiosystems
Conclusion
This study presents a scalable strategy for producing iNK cells from iPSCs, providing a consistent and efficient solution to fulfill clinical needs. By improving methods, 96.33 % of iNK cells were produced by Day 35.
At day 49, growth had exceeded 70 K-fold, with > 90 % viability and significant cytotoxic activity. This technology addresses the availability and variability of NK cells, providing a stable supply for off-the-shelf immunotherapies. It also supports future clinical applications and expanded accessibility in cell-based immunotherapy.
References
- Maskalenko, N.A., Zhigarev, D. and Campbell, K.S. (2022). Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nature Reviews Drug Discovery, 21(8), pp.559–577. DOI: 10.1038/s41573-022-00413-7. https://www.nature.com/articles/s41573-022-00413-7.
- Vivier, E., et al. (2024). Natural killer cell therapies. Nature, 626(8000), pp.727–736. DOI: 10.1038/s41586-023-06945-1. https://www.nature.com/articles/s41586-023-06945-1.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Jan 20, 2026