Diabetes is a worldwide health crisis. Estimates in 2024 revealed 537 million adults aged 20 to 79 are affected by diabetes, and this number is projected to increase to 643 million by 2030.
The disease has multiple forms. Type 1 diabetes accounts for just 5-10 % of cases and is caused by autoimmune destruction of pancreatic β-cells, leading to insulin deficiency. Type 2 diabetes, making up 90-95 % of cases, involves insulin resistance and relative insulin insufficiency, and is often linked to lifestyle factors like inactivity and obesity.
Gestational diabetes and rare forms make things even more complex. Current treatments, including insulin injections, oral hypoglycemic agents, and lifestyle changes, predominantly manage symptoms rather than cure the disease. Long-term insulin use can cause health complications such as hypo and hyperglycemia and cardiovascular and renal issues, emphasizing the need for more effective therapeutic approaches.
In cell therapy, research exploring stem cell-based treatments for diabetes offers new hope. Induced pluripotent stem cells (iPSCs) have unique properties of unlimited self-renewal and multi-lineage differentiation potential, and they have emerged as a focal point in diabetes research. Scientists are currently trying to differentiate iPSCs into pancreatic islet cells capable of secreting insulin, with the aim of them replacing damaged β-cells, fundamentally addressing the issue of insulin deficiency.
Vertex Pharmaceuticals in particular has made progress in this domain. Its product, VX-880 (Zimislecel), has achieved significant breakthroughs, demonstrating impressive efficacy in early clinical trials. Some patients experienced a substantial reduction in insulin requirements and a notable improvement in blood glucose control after treatment, with the product also demonstrating favorable safety and tolerability profiles.
As a result of these findings, the therapy has advanced to Phase III clinical trials and is scheduled for market launch in 2026. VX-880 has received important regulatory designations, including the PRIME (Priority Medicines) designation from the European Medicines Agency (EMA), the Regenerative Medicine Advanced Therapy (RMAT), and Fast-Track status from the U.S. Food and Drug Administration (FDA), highlighting its immense potential to revolutionize diabetes therapy.
Despite these positive developments, the journey of iPSC-based therapies from bench to bedside is facing significant challenges. One of the greatest difficulties is the immune response to transplanted iPSC-derived islet cells. Since they are foreign entities, the cells are at risk of being recognized and attacked by the patient's immune system, in turn requiring immunosuppressive therapy.
While essential for preventing rejection, long-term use of immunosuppressants carries substantial dangers, such as increased cancer risk, kidney damage, and heightened susceptibility to infections. Finding and maintaining a balance between immune suppression and minimizing adverse effects remains a complicated challenge.
Gene editing technologies offer a potential solution for reducing immune rejection by modifying iPSCs to lower their immunogenicity. Yet this method carries its own challenges. Technical limitations pose substantial risks, such as off-target effects that can lead to unintended genomic alterations and potentially harmful consequences like tumorigenesis. Gene editing also raises complicated ethical questions regarding potential eugenic implications, human germline modification, and long-term impacts on future generations, necessitating rigorous regulatory oversight and ethical evaluation.
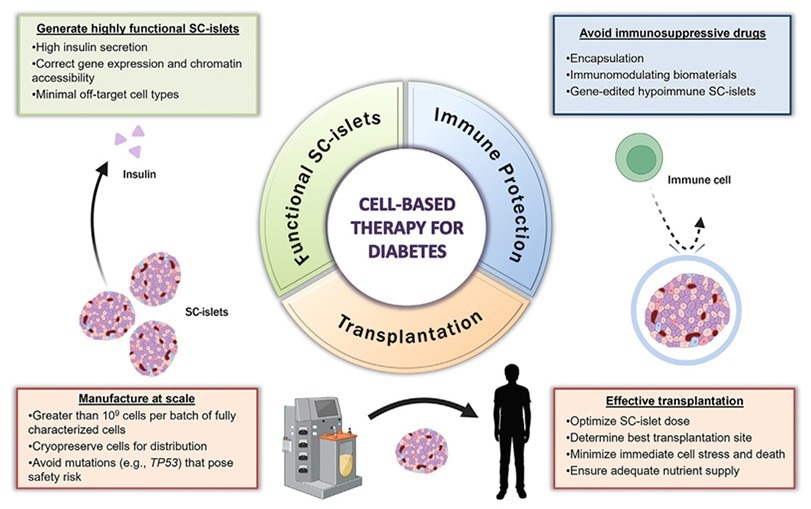
Figure 1. Key pillars of a successful SC-islet therapy for treating T1D. Image Credit: ACROBiosystems
The large-scale production of iPSCs and the generation of functional islet cells also present forbidding challenges. Maintaining cell quality and consistency during the scale-up process is especially challenging because even slight fluctuations in culture conditions, such as pH, nutrient concentration, and temperature, can disrupt cell growth and differentiation.
Additionally, achieving efficient differentiation requires a reasonable cost for commercial viability. Good Manufacturing Practice (GMP)-compliant and cost-effective growth factors are essential for optimizing the differentiation process, reducing costs, and streamlining production, and in turn facilitating the translation of iPSC-derived islet cell therapies into clinical practice.
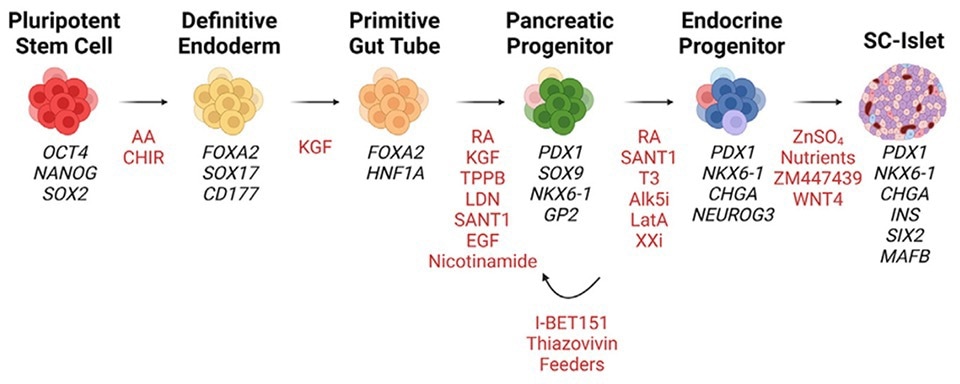
Figure 2. Manufacturing process for during the multistage differentiation of hPSCs to SC-islets. Cell Stem Cell (2023). Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Image Credit: ACROBiosystems
Product highlights
• Activin A (Cat. No. GMP-ACAH37 & ACA-H5314)
Offered in both GMP grade and Premium Grade (PG), this reagent achieves highly efficient endoderm differentiation. It dramatically lowers costs, since using gram-level quantities can help to save millions of dollars in drug production. In the company’s internal verification, Activin A has achieved a differentiation rate of stem cells into definitive endoderm (DE) of approximately 90 %.
Using their expertise in stem cell research and recombinant protein production, ACRO’s novel technology helps control costs and deliver the best Activin A reagent available. Their growth factors have already been used in clinical trials, especially in iPSC-derived islet cell therapy.
• KGF (Cat. No. FG7-H5213)
Engineered for strong, reproducible cell culture performance, it could support at least 95 % differentiation rate of stem cells into pancreatic progenitor cells (PP2).
• ACRO's GMP products hold a substantial cost advantage over Competitor R.
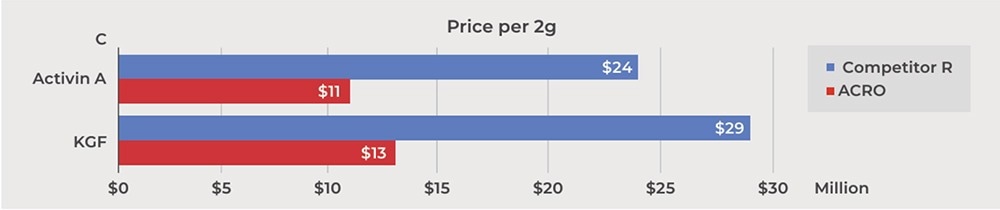
Image Credit: ACROBiosystems
• A complete iPSC-to-mature-islet-cell differentiation model
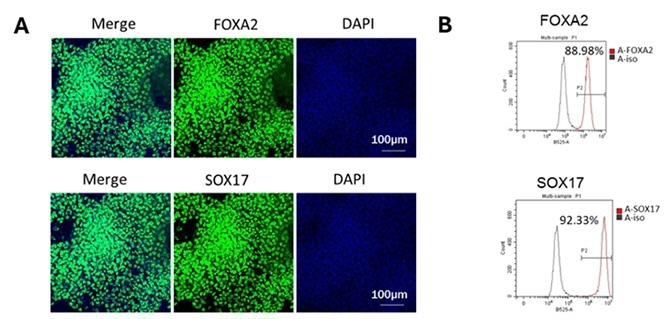
Step 1 – iPSC to DE Differentiation. Image Credit: ACROBiosystems
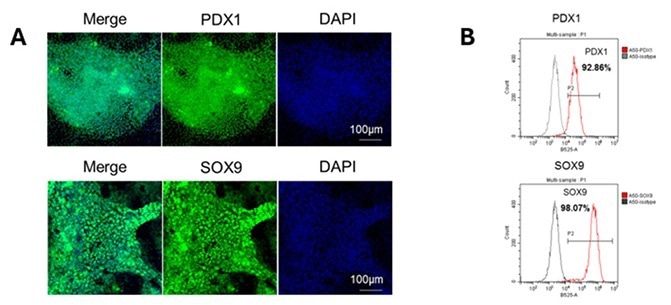
Step 2 – iPSC to pancreatic endoderm differentiation. Image Credit: ACROBiosystems
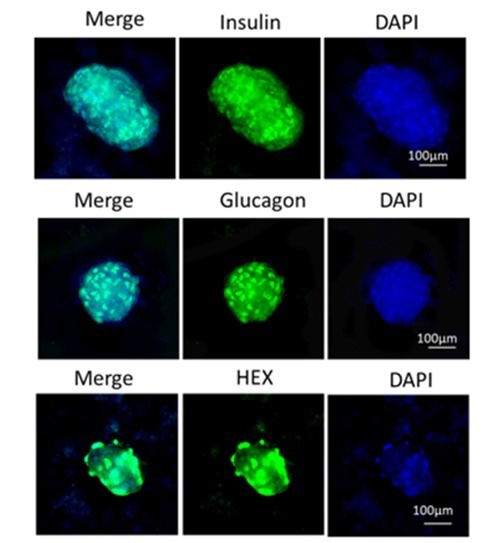
Step 3 – iPSC to mature pancreatic cell differentiation. Image Credit: ACROBiosystems
ACROBiosystems has developed an efficient solution for iPSC-derived islet cell differentiation, achieving a balanced composition of 60 % insulin-producing beta cells, 20 % glucagon-secreting alpha cells, and 20 % HEK-derived delta cells, optimized to replicate native islet function for effective glucose regulation.
As a globally recognized and regulation-compliant supplier, ACRO adheres to international regulatory and legal standards to ensure seamless integration into a client’s manufacturing processes. It offers a full range of customizable services to meet a company’s specific needs, providing comprehensive support to overcome the unique challenges seen in stem cell therapy, driving the success of clients’ projects.
iPSCs have shown great potential to revolutionize diabetes treatment, and overcoming these challenges will be key to realizing their full promise. Continued research, ethical deliberation, and technological innovation are crucial for transforming this scientific breakthrough into a sustainable, accessible, and practical therapeutic solution that can benefit millions of diabetes patients worldwide.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.