Autoimmune drug development increasingly recognizes that the Fc-FcR axis is more than an effector mechanism; it is a controllable immunological node that bridges humoral and cellular responses.
This axis is responsible for two complementary regulatory tasks: Fc gamma receptors mediate effector functions by maintaining a dynamic balance of intracellular "activation" and "inhibition" signals.
The Fc-FcR axis activates Fc gamma receptors (most notably, FcγRIIa and FcγRIIIa) via ITAMs to signal through the Src/Syk pathways. It also drives phagosome formation, actin remodeling, oxidative burst, and transcriptional programs that underpin ADCC, ADCP, and pro‑inflammatory cytokine release.
The inhibitory FcγRIIb also transmits ITIM‑dependent brake signals able to limit B‑cell activation and the responsiveness of effector cells. The functional or genetic loss of FcγRIIb shifts this balance toward pathogenic activation.
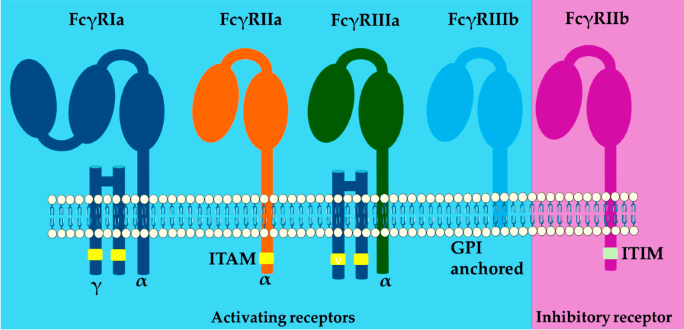
Fc gamma receptors’ structure. Image Credit: https://doi.org/10.3390/ijms26051851
At the same time, the Neonatal Fc Receptor (FcRn) binds to IgG in endosomes’ acidic environment, protecting it from lysosomal degradation and recycling it back into the bloodstream. This function effectively extends the half-life of IgG.
The excessive retention of pathogenic IgG is a key driver of pathology in many antibody-mediated autoimmune diseases, meaning that blocking the FcRn-IgG interaction accelerates pathogenic antibody clearance, creating an efficient and rapid path toward the treatment of autoimmune diseases.
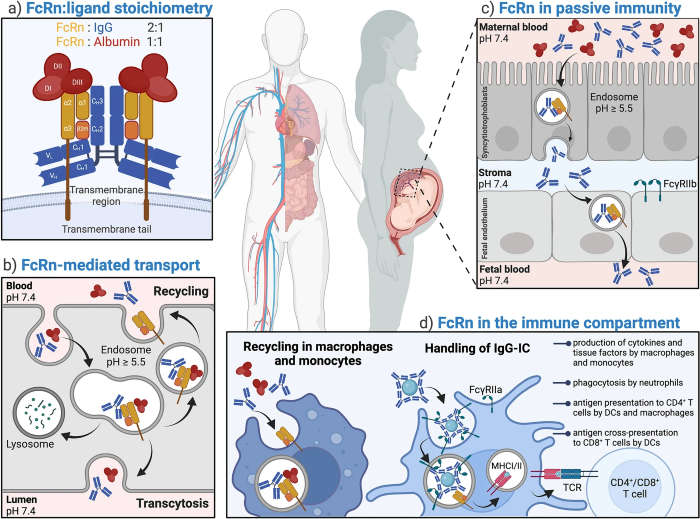
Neonatal Fc receptor (FcRn) biology. Image Credit: https://doi.org/10.1007/s40259-025-00708-2
Therapeutic strategies: Reducing load and recalibrating signaling
Therapeutic approaches tend to be centered around three complementary aims: the rapid reduction of pathogenic IgG, the recalibration of FcγR‑mediated effector function, and the induction of antigen‑specific tolerance.
FcRn inhibitors exemplify the first of these aims, accelerating IgG catabolism and quickly reducing the number of circulating pathogenic antibodies. Clinical validation in antibody-mediated disorders has shown that IgG depletion has the potential to translate into rapid symptomatic benefit when disease activity correlates with antibody burden.
Fc engineering addresses the second of these aims by tuning both pharmacokinetics and effector function. This results in half-life-extending mutations (for instance, YTE or LS) that improve FcRn affinity to prolong exposure.
Engineered Fc variants able to preferentially engage the inhibitory FcγRIIb can potentially blunt B‑cell activation and downstream autoantibody production without significantly impairing host defenses.
Antigen–Fc fusion constructs and glycoengineering are targeted strategies able to induce tolerance or bias effector phenotypes. For example, MOG Fc-style constructs have the capacity to direct antigen delivery to tolerogenic pathways, while sialylation or galactosylation patterns on Fc glycans can shift antibodies toward anti- or pro-inflammatory outcomes, respectively.
Clinical translation: Mapping strategy to indication
The ideal strategy from a translational standpoint depends on successfully mapping dominant disease mechanisms to the simplest effective intervention.
Myasthenia Gravis (MG), Immune Thrombocytopenia (ITP), and other conditions characterized by acute, antibody-mediated tissue injury can often be effectively addressed by FcRn antagonism. This sees the rapid reduction in circulating IgG (validated via regulatory approval from Efgartigimod for MG), translating directly into clinical improvement.
Diseases like Systemic Lupus Erythematosus (SLE), which exhibit more complex immunopathology, generally require combination approaches that can both restore inhibitory signaling and reduce pathogenic IgG load.
SLE’s pathology is driven by ICs activating TLR7/9 (typically exacerbated by defects in the inhibitory Fc gamma RIIb), which means that engineered Fc constructs able to improve Fc gamma RIIb engagement are a sensible part of ensuring immune "signal reset," working alongside FcRn inhibitors.
The early incorporation of mechanism-relevant biomarkers (for example, circulating pathogenic IgG levels, Fc gamma R expression or genotype, Fc glycoform profiles, and downstream signatures such as type I interferon) is key to enabling patient stratification across all indications, as well as delivering early evidence of target engagement.
Conclusion
The Fc–FcR axis offers a range of complementary levers, such as rapid IgG depletion, antigen-specific modulation, and selective signaling recalibration, that can be applied selectively or combined to match disease biology.
Programs beginning with a mechanism-to-indication map before embedding clear biomarker and PD plans, and employing functional assays and validated Fc/FcR reagents are best positioned to efficiently translate these approaches while sufficiently managing immunocompetence and safety risks.
ACROBiosystems’ Fc receptor research portfolio for autoimmune disease research
ACROBiosystems offers an integrated Fc receptor toolbox designed to directly support the rapid advancement of a pipeline while supporting discovery via translational research.
This platform offers a range of high-purity, high-affinity Fc receptor proteins, FACS-verified overexpression cell lines, no-wash TR-FRET binding assay kits, and high-sensitivity, robust signal reporter cell lines for ADCC/ADCP functional validation. These components are also backed by global commercial authorization.
The company also offers the Fc receptor protein panel to help streamline required multi-receptor synergistic verification.
This convenient solution integrates the key FcRs (for instance, FcRn/Fc gamma RI/IIa/IIb/IIIa) required for screening and validation, ensuring optimized cost and time while guaranteeing consistent quality (confirmed via SDS-PAGE and SEC-MALS) and functional assurance (verified via SPR/BLI/ELISA).
References and further reading
- Sepúlveda-Delgado, J., Llorente, L. and Hernández-Doño, S. (2025). A Comprehensive Review of Fc Gamma Receptors and Their Role in Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 26(5), p.1851. DOI: 10.3390/ijms26051851. https://www.mdpi.com/1422-0067/26/5/1851.
- Takai, T. (2002). Roles of Fc receptors in autoimmunity. Nature Reviews Immunology, (online) 2(8), pp.580–592. DOI: 10.1038/nri856. https://www.nature.com/articles/nri856.
- Gjølberg, T.T., et al. (2025). Targeting the Neonatal Fc Receptor in Autoimmune Diseases: Pipeline and Progress. BioDrugs, 39(3), pp.373–409. DOI: 10.1007/s40259-025-00708-2. https://link.springer.com/article/10.1007/s40259-025-00708-2.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.