The development of new drugs for Alzheimer's disease (AD) has faced repeated hurdles over the past decade.
Monoclonal antibodies targeting amyloid-β (Aβ) have exhibited the capacity to reduce plaque load in the brain in recent large-scale clinical trials, demonstrating a trend toward slowing cognitive decline in some patients with early-stage AD. This development marks a significant step forward in the clinical validation of the anti-Aβ therapeutic strategy.
The 2025 Clinical Trials on Alzheimer's Disease (CTAD) conference saw discussions on this treatment gradually shift from questions around whether the strategy is effective to more nuanced discussions around systematically improving the treatment’s safety and implementation efficiency based on its established efficacy. This helps ensure the needs of long-term clinical practice are better met.
Plaque removal breakthroughs and challenges
Multiple clinical studies have highlighted how the sustained reduction of cerebral Aβ levels can slow disease progression in some patients. These findings have become the foundation for AD-related drugs’ clinical application.
A number of presentations at CTAD 2025 examined the challenges that current anti-Aβ therapies still face in the real world, most notably in terms of amyloid-related imaging abnormalities (ARIA), such as microhemorrhages (ARIA-H) and cerebral edema (ARIA-E).
ARIA occurrences influence antibodies’ dosing selection and administration strategies while considerably increasing the complexity of clinical management and radiographic monitoring.
As the efficacy of anti-Aβ strategies continues to be validated, the field is beginning to enter a new research phase focused on determining how to better balance therapeutic benefits and safety while maintaining plaque clearance capability. This work has become a core issue that requires urgent resolution.
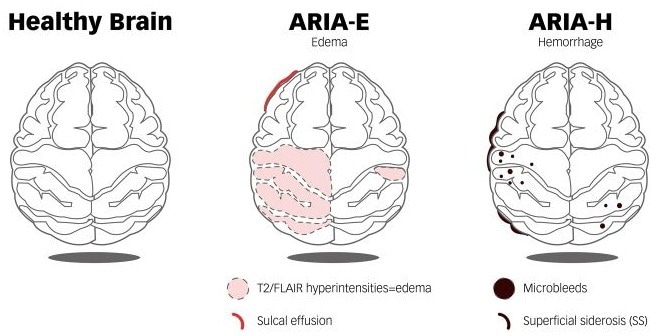
MRI Comparison Between a Healthy Brain and a Brain with ARIA-E and ARIA-H. Image Credit: ACROBiosystems
BBB shuttle technology: Optimizing delivery pathways as a novel strategy to mitigate ARIA risk
Conventional anti-Aβ monoclonal antibodies typically rely on passive diffusion to enter the central nervous system. High peripheral dosing is often required to achieve sufficient brain exposure and plaque-clearing efficacy, but this can contribute to an increased ARIA risk.
Blood-brain barrier (BBB) shuttle technology has garnered considerable attention in recent years. Most notably, mechanisms using transferrin receptor (TfR)-mediated transcytosis represent a novel antibody delivery pathway.
The use of engineered antibody molecules with optimized BBB shuttle modules can improve distribution efficiency within the brain parenchyma, achieving this without significantly increasing systemic exposure.
Current research suggests that these BBB-shuttled antibodies accomplish higher brain exposure while simultaneously demonstrating comparatively lower binding to cerebrovascular amyloid angiopathy (CAA) versus traditional antibodies.
It is theorized that this distribution profile, which favors parenchymal delivery over vascular binding, can help lower the likelihood of vessel-related injury and reduce the risk of ARIA.
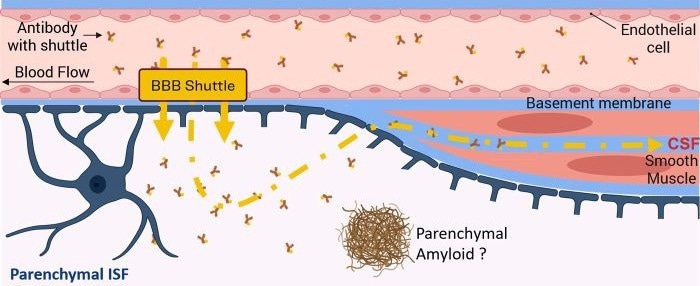
Schematic Diagram of Antibody Delivery to Brain Parenchyma via BBB Shuttle Technology. Image Credit: ACROBiosystems
Research progress and pipeline landscape
Data presented by Dr. Ronald DeMattos from Eli Lilly at the CTAD 2025 conference compared Trontinemab (a next-generation antibody based on gantenerumab that has been fused with a TfR shuttle module) against traditional gantenerumab.
The data presented showed that Trontinemab attained a higher plaque clearance rate within a comparatively shorter treatment period. ARIA-E incidence associated with Trontinemab was lower within the disclosed data range than historically reported rates for traditional gantenerumab studies. This represented a more promising overall benefit-risk profile.
Several pharmaceutical companies worldwide are currently developing next-generation anti-Aβ antibody pipelines, exploring a range of BBB shuttle technology platforms, including CD98, TfR, and INSR.
In vivo distribution characteristics and delivery pathways are becoming increasingly important considerations in the design of differentiated anti-Aβ therapies.
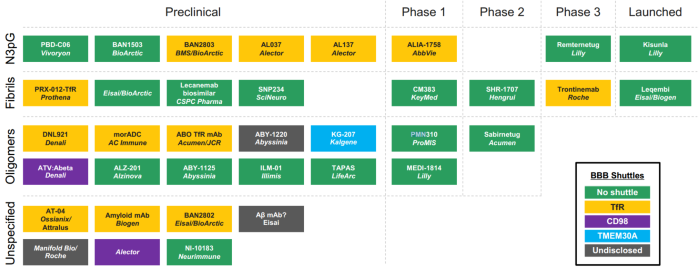
Pipeline of Anti-Aβ Antibody Drug Development. Image Credit: ACROBiosystems
Conclusion
The overarching message from CTAD 2025 is clear: the research focus of anti-Aβ therapeutics is gradually shifting from "plaque removal" to "optimizing delivery pathways and in vivo distribution."
BBB shuttle technology represents a promising new direction for exploring the potential to reduce ARIA risk and improve brain delivery efficiency. This technology is already impacting design strategies for the next generation of anti-Aβ antibodies.
It is anticipated that future differentiation and competition in this field will depend more on attaining a superior balance between safety and efficacy.
Innovative solutions from ACROBiosystems
As the ACROBiosystems brand focused on neuroscience, Aneuro delivers a range of cutting-edge solutions designed to accelerate AD research and drug development, including pre-formed fibrils (PFFs), stable cell lines, target proteins, and p-tau antibodies.
Learn More About AD-Related Proteins. Source: ACROBiosystems


Image Credit: ACROBiosystems
References and further reading
- DeMattos, R. B. (2025). Next Generation Anti-Amyloid Therapies.....Shuttling Past the ARIA Risk. Presentation at the Clinical Trials on Alzheimer's Disease (CTAD) conference.
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.