Antibody-Drug Conjugates (ADCs) are a widely used and prominent class of targeted therapeutics, especially in cancer treatment.
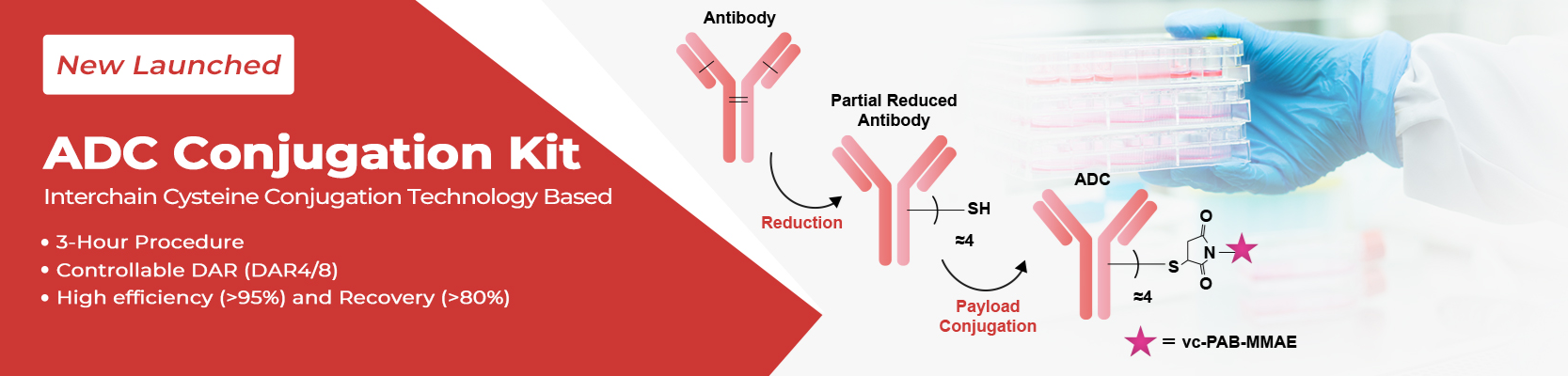
Image Credit: ACROBiosystems
These complex biomolecules are specifically engineered to selectively deliver therapeutic agents to target cells by linking them to monoclonal antibodies. The safety profile and effectiveness of ADCs are impacted by the selection of their components, such as the antibody, therapeutic agent, and linker. They are also significantly affected by the chemical method used for their conjugation.
ADC development currently utilizes two primary strategies for the attachment of therapeutic agents to antibodies: site-specific conjugation and random (stochastic) conjugation.
The selected method considerably affects the resulting ADC's stability, pharmacokinetic (PK) properties, homogeneity, and overall therapeutic index.
The random conjugation method uses naturally occurring reactive residues on the antibody to streamline ADC preparation and reduce technical barriers to a considerable degree.
Its key features of rapid reaction kinetics, native compatibility, and controllable efficiency are in close alignment with the core demands of early-stage ADC development, including fast iteration, the availability of multiple batches, and low cost.
Random conjugation methods have been widely adopted in commercial ADCs like Adcetris and Kadcyla as a result of their robustness for manufacturing. These methods generally target naturally occurring amino acid residues.
Lysine conjugation uses the epsilon-amine groups of lysine residues. There is an abundance of these residues on the antibody surface. Amide bonds are formed via this approach, but the large number of potential conjugation sites can lead to a heterogeneous mixture with variability in the Drug-to-Antibody Ratio (DAR).
Cysteine conjugation targets free thiol groups (-SH) produced by reducing the antibody’s interchain disulfide bonds. This approach offers more control over the DAR versus lysine conjugation, but conjugates formed using maleimide chemistry at these sites may be susceptible to deconjugation in plasma as a result of thiol exchange reactions with albumin. This can limit the ADC’s stability.
Site-specific conjugation strategies, on the other hand, produce a more homogeneous ADC population with a defined attachment point and a precise DAR. This approach is typically anticipated and expected to result in more predictable PK profiles, enhanced stability, and potentially a wider therapeutic window.
Engineered Cysteine (THIOMABs) involves the use of genetic engineering to introduce defined cysteine mutations at carefully selected sites on the antibody. This approach allows precise control over the location and number of conjugation points.
Enzymatic methods utilize enzymes, such as microbial transglutaminase (mTGase), which can recognize specific engineered peptide sequences on the antibody to catalyze conjugation at defined residues.
Non-Natural Amino Acid (nnAA) incorporation is a sophisticated technique that uses genetic code expansion to incorporate distinct chemical handles (for instance, azides) into the antibody structure. These handles can then be used for extremely efficient, bioorthogonal "click chemistry" conjugation.
Site-specific conjugation boasts reduced off-target toxicity and high homogeneity, making it ideally suited to large-scale manufacturing and late-stage development.
Non-site-specific conjugation, on the other hand, emphasizes flexibility, speed, and broad applicability, addressing the key need for scenario adaptability and efficiency in early-phase research.
Each of these methodologies offers specific benefits that make them better suited to distinct stages of ADC research and development.
Employing the most appropriate conjugation technology is key to ensuring successful ADC design and development, whether this involves initial high-throughput screening or later-stage clinical optimization.
Optimized cysteine conjugation for rapid screening
ACROBiosystems has introduced its ADC Conjugation Kit, which delivers a precise, rapid, and robust ADC preparation process. These kits, based on interchain cysteine conjugation technology, are designed to accelerate the research cycle within this evolving landscape.
The ADC Conjugation Kit reduces antibody disulfide bonds to generate reactive cysteine residues for non-site-specific maleimide conjugation. This classic approach facilitates rapid, controlled ADC preparation, requiring just three hours to acquire ADC conjugates with homogeneous DAR.
Conjugates created via this approach exhibit significant cytotoxicity, making them a robust tool for mechanistic studies and ADC screening.
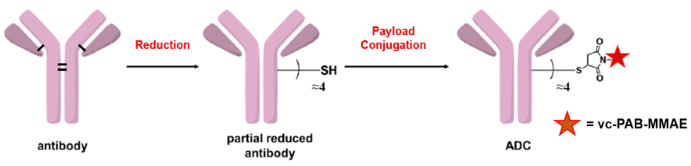
Image Credit: ACROBiosystems
Product features
ACROBiosystems’ ADC Conjugation Kits are:
- Rapid: Complete ADC preparation in under three hours
- Precise: Controlled DAR 4/8 with preserved antibody activity
- Efficient: Offering more than 95 % conjugation and more than 80 % recovery
- Low input: Compatibility with ≥ 2 mg/mL antibodies
- Simple: 15-minute purification via spin column
Protocol overview
The kits’ protocol is simple and straightforward:
- Prepare the antibody solution for 2 mg/mL in 1×PBS buffer (pH 7.2-7.4)
- Complete antibody reduction at 37 °C for 1 hour
- Perform antibody conjugation at 23 °C for 1 hour
- Complete reaction quenching RT for 0.5 hours
- Perform a purification and buffer change
Validation data
The ADC products presented here were prepared using the ADC Conjugation Kit (MMAE, DAR4, 200 μg, for human IgG1, Catalog No. ADC-P013).
Antibody resource: Trastuzumab biosimilar
DAR and purity validation via HPLC (HIC + SEC)
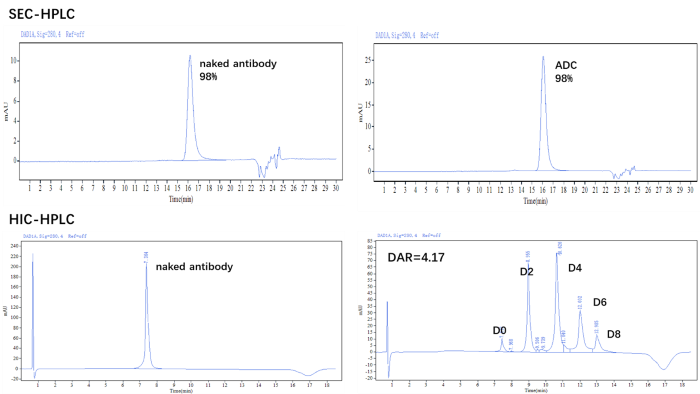
Figure 1. The ADC was prepared using the ADC Conjugation Kit (MMAE, DAR4) and analyzed by HIC and SEC-HPLC. The average drug-antibody ratio (DAR) is 4.0 ± 0.5, and the purity of the ADC is greater than 95 %. Image Credit: ACROBiosystems
In vitro cytotoxicity validation
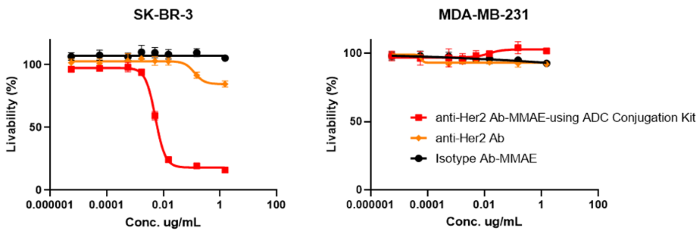
Figure 2. In vitro cytotoxicity assays: The ADC can bind and internalize in target cells (SK-BR-3) with high expression of Her2 and release MMAE inside the cells to induce a cytotoxic effect (IC50=0.0058 µg/mL). Meanwhile, no cytotoxicity was observed in Her2 receptor-negative cell lines (MDA-MB-231). Image Credit: ACROBiosystems
Acknowledgments
Produced from materials originally authored by ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.