Obesity is now a global health challenge, catalyzed by changes in dietary and lifestyle patterns. As well as changing external appearance, obesity increases the risk of diabetes, cancer, mental health conditions, cardiovascular diseases, and neurodegenerative disorders.
This health crisis demonstrates the need for more research to be conducted into the etiology of obesity and regulatory mechanisms to develop evidence-based prevention strategies and therapeutic interventions for associated comorbidities.
Health risks tied to obesity – body mass index. Image Credit: https://www.bmisurgery.com/health-risks-tied-to-obesity-2
Mechanisms of obesity: From energy imbalances to multidimensional metabolic dysregulation
Obesity pathogenesis involves complicated interactions across energy metabolism dysregulation, chronic inflammation, and adipose tissue dysfunction. At the core of this is a resolute surplus of energy, leading to the pathological expansion of white adipose tissue (hyperplasia or hypertrophy) and a diminished thermogenic capacity in brown adipose.
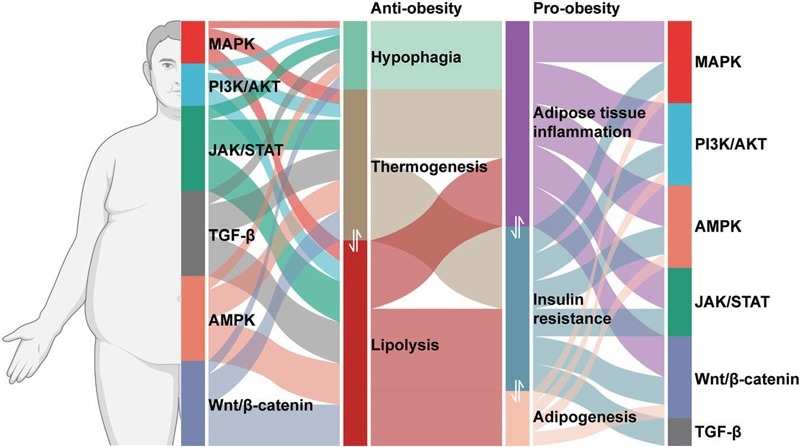
Signaling pathways involved in pro-obesity and anti-obesity mechanisms. Image Credit: https://doi.org/10.1038/s41392-022-01149-x
Uncontrolled adipocyte accumulation triggers endocrine dysregulation, which is characterized by the abnormal secretion of adipokines, including leptin, resistin, and adiponectin. Leptin resistance manifests as defective hypothalamic JAK2/STAT3 signaling, which in turn disrupts appetite regulation. Reduced adiponectin levels act differently, impeding the AMPK/PGC-1α pathway-mediated fatty acid oxidation.
Chronic inflammation is a pivotal mechanism through which macrophage infiltration in adipose tissue activates TLR4/NF-κB signaling to release pro-inflammatory cytokines (TNF-α, IL-6), in turn stimulating JNK and IKKβ signaling nodes. This cascade induces serine phosphorylation of insulin receptor substrate 1, thereby hindering the signaling of PI3K/Akt in response to insulin and promoting systemic insulin resistance.
Concurrently, the overload of nutrients triggers mTORC1 signaling to enhance lipid synthesis via SREBP-1c while repressing autophagy. These successive abnormalities within signaling networks establish a vicious cycle of metabolic dysregulation, ultimately resulting in complications like cardiovascular diseases and type 2 diabetes.
Obesity therapeutics: The rise of multi-target synergy
• GLP-1R agonists: Pioneering single- to multi-target strategies
GLP-1R agonists command the landscape of weight management therapeutics. Novo Nordisk's semaglutide (Wegovy), a single-target GLP-1R agonist, has demonstrated a 15 % weight reduction over a 68-week treatment period, achieving sales of over $7 billion USD in 2023. Eli Lilly's tirzepatide (Mounjaro®), a dual GLP-1R/GIPR agonist, demonstrated superior efficacy and generated 22.510 billion. Lilly's retatrutide, a novel triple agonist targeting GLP-1R/GlPR/GCGR receptors, achieved 24 % weight reduction in Phase 1 studies, and it is thought that it will become the first FDA-approved triple-target weight loss medication by 2025.
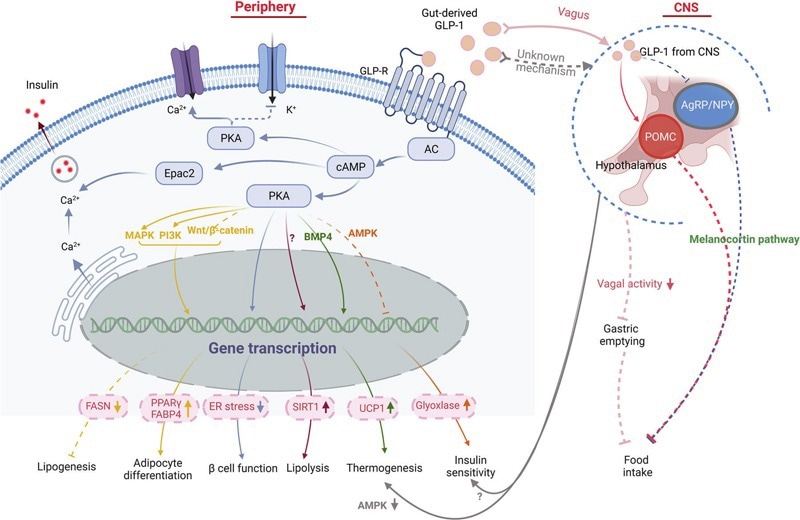
GLP-1 signaling pathway in obesity pathogenesis. Image Credit: https://doi.org/10.1038/s41392-022-01149-x
• Emerging targets: Unlocking new horizons in obesity therapeutics
The full-length G protein-coupled receptor 75 (GPR75), a hypothalamus-specific GPCR, exercises regulatory control over energy homeostasis and appetite doubly via CCL5/RANTES-mediated activation. Upon binding with a ligand, GPR75 couples with heterotrimeric G-proteins to trigger inositol trisphosphate/calcium signaling cascades, modulating activity in NPY/AGRP/GABA co-expressing neurons, which are critical for metabolic balance.
A landmark study demonstrated that individuals with GPR75 truncating mutations exhibit a 1.8 kg/m² reduction in BMI and a 54 % lower risk of obesity. Preclinical validation demonstrated that genetic knockout confers resistance to high-fat, diet-induced obesity and enhances glucose metabolism, underscoring GPR75's dual mechanistic role - suppressing hyperphagia and optimizing energy expenditure at the same time - as a new anti-obesity therapeutic target with transformative translational potential.
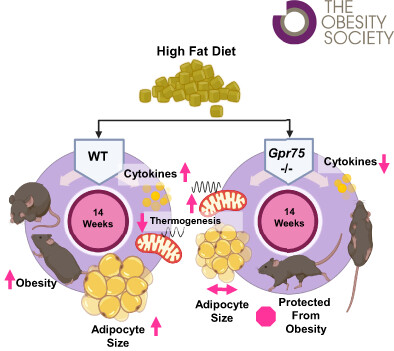
GPR75 knockout mice exhibit reduced food intake under high-fat diet conditions. Image Credit: https://doi.org/10.1002/oby.23692
Bimagrumab, developed by Eli Lilly, is a fully human monoclonal antibody that selectively targets activin type II receptors (ActRIIA/B) to block the signaling pathways that are mediated by myostatin and activins. It reduces myostatin-mediated protein degradation by inhibiting Smad2/3 phosphorylation, in turn increasing lean muscle mass and enhancing fat metabolism.
Its dual efficacy has been demonstrated in both preclinical and Phase II clinical trials, in which a 12.3 % increase in muscle volume and significant body recomposition were seen (lean body mass +8.5 % and fat mass -6.2 %).
This novel mechanism positions Bimagrumab as a possible first-of-its-kind therapeutic to address unmet needs in combination with muscle-metabolism therapies, with great potential for sarcopenia and obesity management.
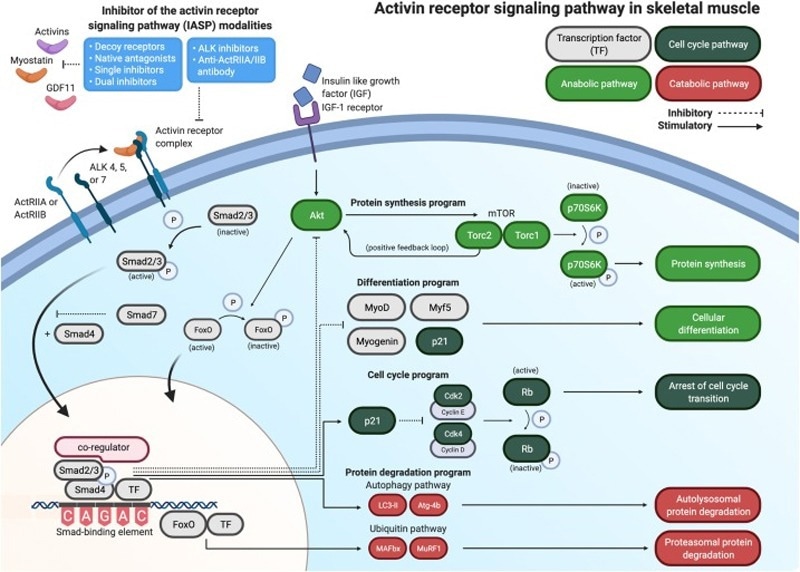
A map of the activin receptor signaling pathway as we currently know it in skeletal muscle. Image Credit: https://doi.org/10.1016/j.cytogfr.2021.04.001
GDF-15, part of the TGF-β superfamily, suppresses appetite and reduces food intake by binding to the hindbrain-specific receptor GFRAL and activating RET kinase signaling. This pathway is distinct from traditional appetite-regulatory systems that make use of ghrelin or leptin.
Human genetic studies confirm the association of the GDF15-GFRAL axis with obesity, while its anti-inflammatory effects, mediated by mechanisms not yet fully understood, expand its therapeutic potential for metabolic diseases.
GDF-15 has emerged as a key therapeutic target for managing type 2 diabetes, obesity, cancer cachexia, and non-alcoholic fatty liver disease (NAFLD) due to its unique dual abilities to suppress appetite and modulate inflammation. These multifaceted abilities suggest GDF-15-based therapies could overcome the limitations of previous approaches and offer a crucial change in the management of complex metabolic-inflammatory comorbidities.
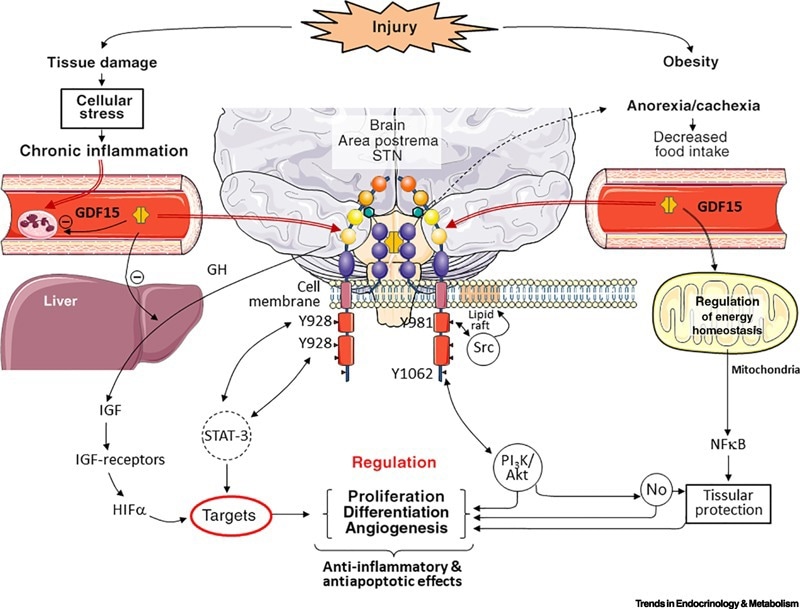
GDF15 is a signal in the organism. Image Credit: https:/doi.orgy10.1016/j.tem.2020.10.004
FGF-21, a hepatic secretory hormone central to metabolic regulation, has emerged as a key biomarker for obesity, with serum levels correlated positively with visceral adiposity and BMI. These levels dynamically reflect both the efficacy of therapeutic weight loss and the severity of obesity.
Mechanistically, FGF-21 sets up dual metabolic modulation by suppressing appetite through hypothalamic signaling, while also catalyzing thermogenesis of adipose tissue and white adipose browning, ‘reducing caloric intake + enhancing energy expenditure’.
In addition, it shows clear therapeutic benefits in ameliorating insulin resistance, dyslipidemia, and hyperglycemia. Preclinical studies have highlighted the therapeutic promise of recombinant FGF-21 analogs, which achieve a 10-15 % reduction of body weight in animal models, while comprehensively reversing metabolic dysregulation. These findings suggest FGF-21-based therapies could be transformative candidates for addressing multifactorial obesity-related pathologies.
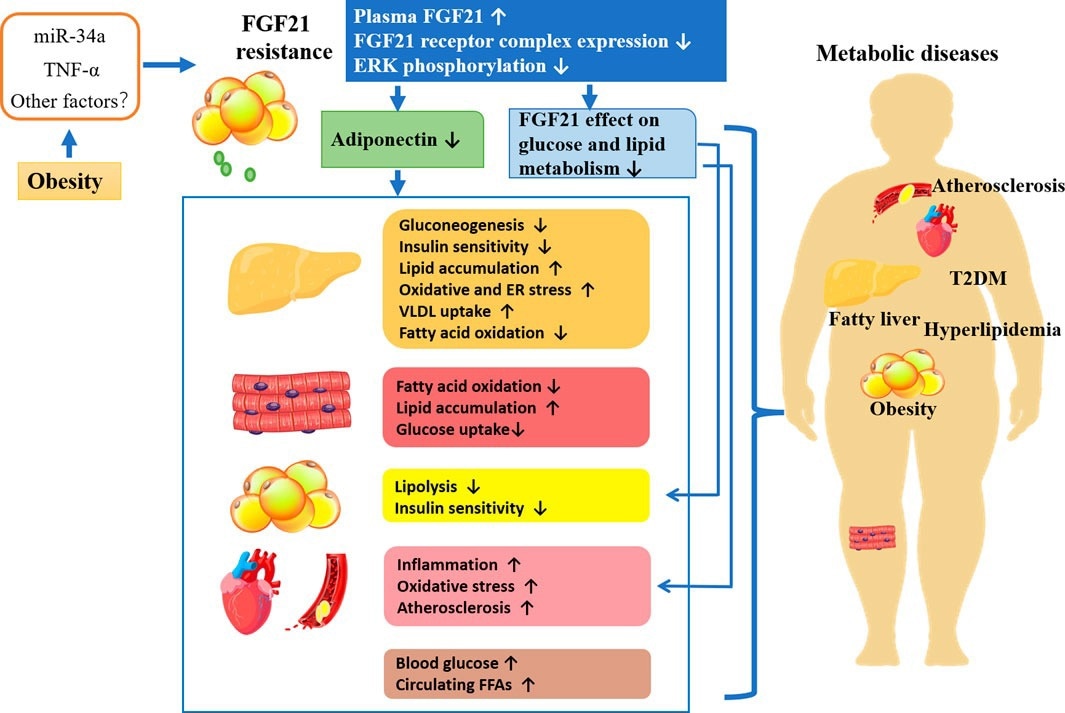
FGF21 resistance and the potential mechanisms underlying metabolic diseases. Image Credit: https://doi.org/10.3389/fphar.2022.1089214
ACROBiosystems uses cutting-edge solutions to advance obesity therapeutics
The company has developed a comprehensive portfolio of recombinant proteins and functional cell lines targeting key receptors in weight management pathophysiology, including GLP-1R, GCGR, GIPR, GPR75, GDF-15, FGF-21, and Activin R.
These solutions support the advancement of targeted therapeutic development in obesity treatment, enabling researchers to accelerate the discovery and optimization of precision medicines and intervention strategies for metabolic disorders.
References
- Wen, X., et al. (2022). Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduction and Targeted Therapy, [online] 7(1). DOI: 10.1038/s41392-022-01149-x. https://www.nature.com/articles/s41392-022-01149-x.
- Hossain, S., et al. (2023). Gpr75 ‐deficient mice are protected from high‐fat diet–induced obesity. Obesity, 31(4), pp.1024–1037. DOI: 10.1002/oby.23692. https://onlinelibrary.wiley.com/doi/10.1002/oby.23692.
- Chen, Z., et al. (2022). The potential function and clinical application of FGF21 in metabolic diseases. [online] 13. DOI: 10.3389/fphar.2022.1089214. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.1089214/full.
- Lodberg, A. (2021). Principles of the activin receptor signaling pathway and its inhibition. Cytokine & Growth Factor Reviews, [online] 60, pp.1–17. DOI: 10.1016/j.cytogfr.2021.04.001. https://www.sciencedirect.com/science/article/pii/S1359610121000344?via%3Dihub.
- Koenen, M., et al. (2021). Obesity, Adipose Tissue and Vascular Dysfunction. Circulation Research, 128(7), pp.951–968. DOI: 10.1161/circresaha.121.318093. https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.121.318093.
- Rochette, L., et al. (2020). Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets. Trends in Endocrinology & Metabolism, 31(12), pp.939–951. DOI: 10.1016/j.tem.2020.10.004. https://www.cell.com/trends/endocrinology-metabolism/fulltext/S1043-2760(20)30201-0.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond.
By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empowers scientists and engineers dedicated to innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.