This article is based on a poster originally authored by Anusha Gupta, Hannah Grice, Chloe Whiting, Oliver Birch, Fiona Leslie, and Megan Webster.
Small airways in health and disease
The small airways serve as a primary line of defense against inhaled particles and facilitate gas exchange by directing air to the alveoli.
These small airways are critical in the pathophysiology of various diseases and are often the initial sites of obstruction and inflammation in conditions such as bronchiolitis, asthma, obliterans, and chronic obstructive pulmonary disease.
The capacity to study small airway physiology in vivo is constrained by their diminutive size and deep location within the lungs, making them challenging to visualize and access using noninvasive imaging techniques.
Newcells Biotech has developed a sophisticated multicellular system to facilitate the study of small airway physiology and disease in vitro.
Primary human basal cells are expanded in culture flasks before being seeded onto semi-permeable inserts under submerged conditions.
Upon reaching confluency, the cells are 'airlifted'—the apical medium is removed—to establish air-liquid interface (ALI) culture conditions. This promotes epithelial polarization and cell differentiation to create a heterogeneous population of epithelial cells that accurately represents the small airways.
Epithelial barrier integrity
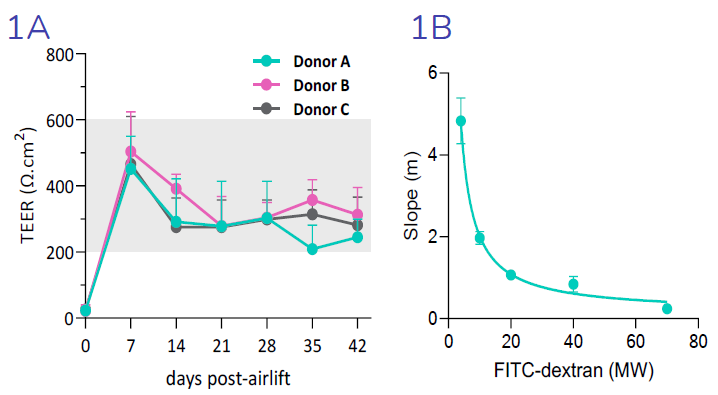
(1A) Transepithelial electrical resistance (TEER), an indicator of epithelial barrier integrity, is increased 7- days post-airlift, confirming epithelial polarization and establishment of epithelial junctions. TEER is reduced from day 7 to day 14, in line with increased cellular ion transport. TEER is maintained from day 14 through to day 42. (1B) Size selectivity of the epithelial barrier is confirmed by decreased flux of FITC-dextran molecules of increasing molecular weight. Image Credit: Newcells Biotech
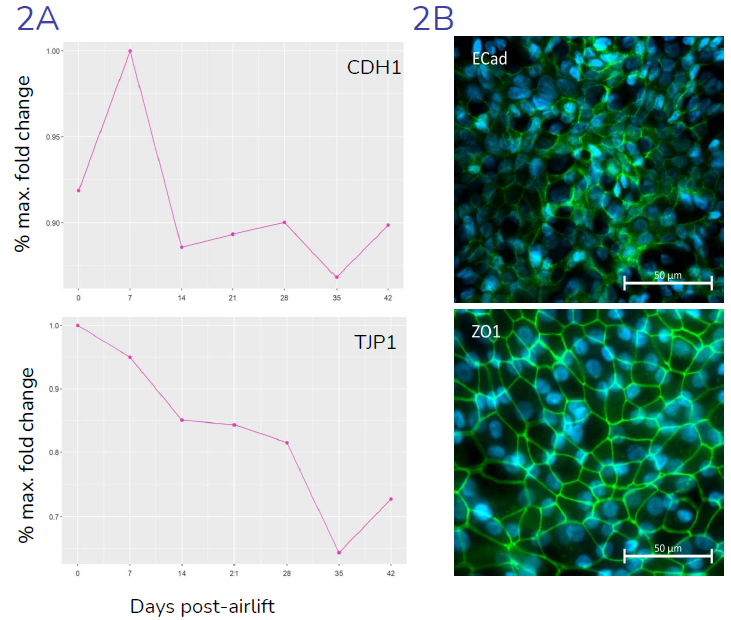
(2A) Gene expression (relative to maximal expression) of key junctional genes CDH1 (E-Cadherin) and TJP1 (ZO-1), throughout the differentiation period, from day 0 (day of air-lift) to day 42, indicating presence of epithelial junctions, in-line with literature data. (2B) Representative images showing expression, and localization of ZO1 and E-Cadherin proteins at epithelial cell: cell junctions. Image Credit: Newcells Biotech
Model validation
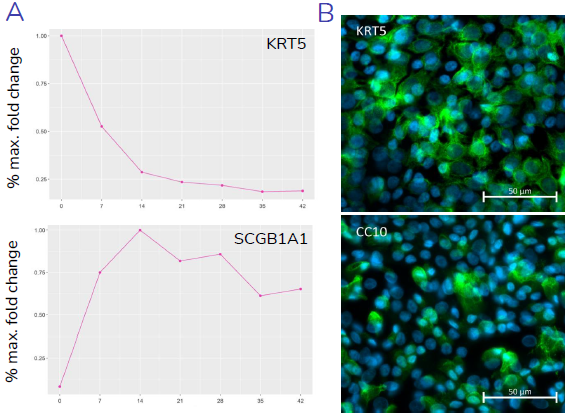
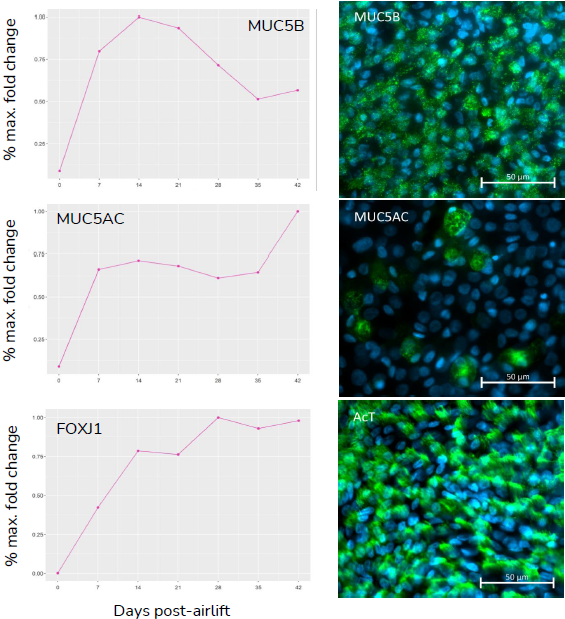
(A) Gene expression data shows relative expression of epithelial specific genes, KRT5, SCGB1A1, MUC5B/MUC5AC, and FOXJ1 throughout the 28-day differentiation period, with changes in expression reflecting differentiation of basal, club, goblet and ciliated cells, respectively. (B) Presence of key epithelial cell types, basal, club, goblet and ciliated cells, at 28-days post-airlift is confirmed by immunocytochemical detection of KRT5, CC10, MUC5B/MUC5AC and acetylated-Tubulin (AcT) respectively. Image Credit: Newcells Biotech
Cytokine-induced damage
Increased permeability of the epithelial layer due to compromised barrier integrity is a hallmark of lung disease.
Tumor Necrosis Factor-α (TNFα) has been reported to be elevated in lung diseases. Cells were stimulated basolaterally with TNFα at specified concentrations for 72 hours, and a dose-dependent response in epithelial breakdown was observed.
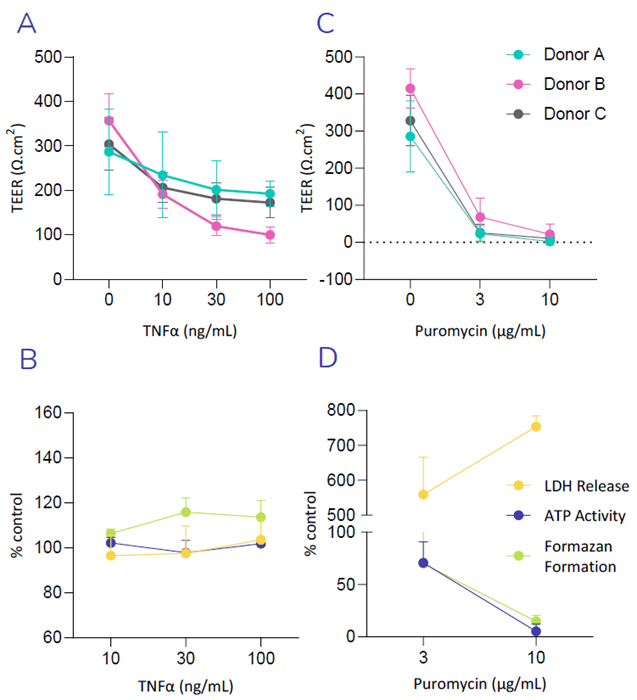
(A) Exposure of Small Airway Epithelial Cells (SAEC’s) to increasing concentration of the TNFα reduces TEER, in-line with cytokine induced breakdown of the epithelial barrier.
(B) No significant increase in LDH release or decrease in ATP- or MTT- activity (formazan formation), was observed following exposure of SAEC’s to TNFα, suggesting TNFα induced breakdown of the epithelial barrier was not observed as a result of cellular toxicity. (C) Puromycin, an antibiotic with known toxic side effects in the lung, was used as a positive control. A decrease in TEER is triggered with increasing concentrations of Puromycin, indicating a loss in barrier integrity. (D) High increase in LDH release as well as a decrease in ATP- and MTT- activity was observed following exposure of SAEC’s to Puromycin, indicating drug induced cellular toxicity. Image Credit: Newcells Biotech
In development
1. Modeling idiopathic pulmonary fibrosis (IPF) in small airway epithelial cells (SAECs)
Following treatment of differentiating SAECs with varying cytokine mixes for 12 days, an increase in epithelial damage was observed, as evidenced by a reduction in (1A) transepithelial electrical resistance (TEER) and an increase in (1B) FITC dextran permeability.
The cytokine-induced damage to the epithelial barrier was not attributed to cytotoxicity, as (1D) LDH release did not increase.
2. Ciliary beat frequency
After treating differentiating SAECs with various cytokine mixes for 10 days (1C), ciliary beat frequency decreased compared to the control.
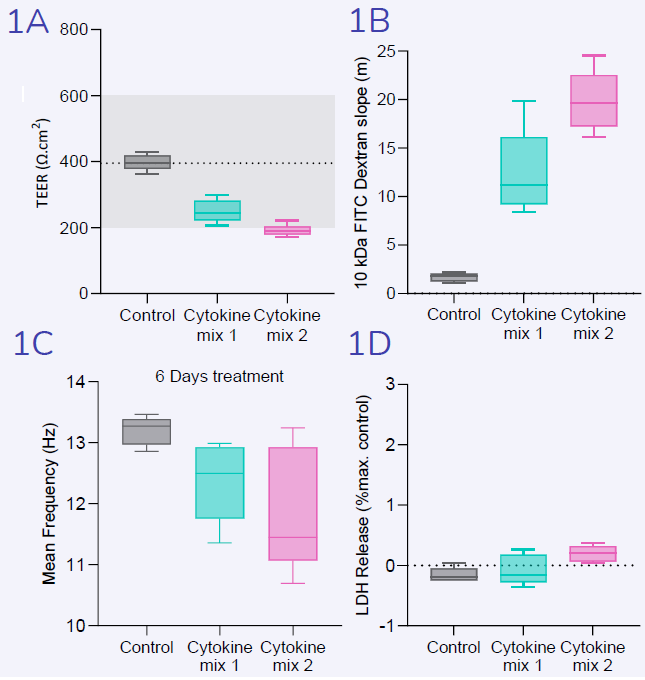
Image Credit: Newcells Biotech
Newcells Biotech’s SAEC model
Model features:
- Three validated primary basal cell donors
- Fully differentiated and multicellular
- Optimized under Air-Liquid Interface conditions
Available analytical techniques and data outputs:
- Epithelial barrier integrity: TEER and FITC-dextran flux measurements
- Gene and protein expression: qPCR and immunocytochemistry
- Epithelial damage studies: cytokine-induced barrier breakdown
- Cellular toxicity assessment: LDH release and cellular metabolism
- Biomarker analysis: ELISA-based detection of secreted factors
Acknowledgments
Produced from material originally authored by Anusha Gupta, Hannah Grice, Chloe Whiting, Oliver Birch, Fiona Leslie, and Megan Webster from Newcells Biotech.
About Newcells Biotech
Newcells Biotech develops in vitro cell-based assays for drug and chemical discovery and development.
Using our expertise in induced pluripotent stem cells (iPSCs), cellular physiology, and organoid technology, we build models that incorporate the “best biology” for predicting in vivo behavior of new drugs.
Our experts have developed and launched assays to measure transporter function, safety, and efficacy in a range of cell and tissue types, including kidney, retina and lungs.
We have the capability to develop and implement protocols to measure cilia beat frequency and toxicity on small airway epithelial cells model, retinal toxicity and disease modelling on retinal organoids and retina epithelium, as well as drug transport in the kidney, DDI and nephrotoxicity across human and a range of preclinical species.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: May 13, 2025