This article and associated images are based on a poster originally authored by Leif, F., Hsia, O., Cowan, A.D., Iso, K. and Ciulli, A. and presented at ELRIG Drug Discovery 2025 in affiliation with BMG LABTECH and University of Dundee.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Targeted protein degradation (TPD) is accomplished by the induced proximity of an E3 ubiquitin ligase and a target protein to promote target ubiquitination and subsequent proteasomal degradation. TPD is an emerging therapeutic tool with high potential to tackle disease-causing proteins.
In the past, TPD has been achieved either by using PROteolysis-TArgeting Chimeras (PROTACs) - bifunctional compounds composed of two separate moieties that individually bind the target and E3 ligase - or via molecular glues that monovalently bind either the ligase or the target.
Herein, the team highlights the mechanism of action of novel bifunctional degraders of BRD4, termed Intramolecular Bivalent Glues (IBGs), introducing a new modality in targeted protein degradation. Instead of connecting target and ligase in trans as PROTACs do, IBGs simultaneously engage and connect two adjacent domains of the target protein in cis, thereby enhancing surface complementarity with E3 ligases.
This conformational change ‘glues’ BRD4 to the E3 ligase DCAF16, utilizing the intrinsic target–ligase affinities, leading to the degradation of BRD4 that does not occur in the absence of the compound. Structural insights into the ternary BRD4–IBG1–DCAF16 complex guided the rational design of further improved degraders of low picomolar potency.
Assay principle
Ternary complex formation involving BRD4 and DCAF16 is evaluated with a Time-resolved Fluorescence Resonance Energy Transfer (TR-FRET) complex-formation assay. This is achieved by using a europium-labeled antibody against BRD4 and Cy5-labeled DCAF16.
If an IBG molecule induces the formation of a ternary complex between the labeled BRD4 and DCAF16 molecules, the fluorophores are in sufficient proximity to form a FRET pair. Thus, exciting the europium donor fluorophore will result in energy transfer, and the fluorescence of the Cy5 acceptor can be measured with a microplate reader.
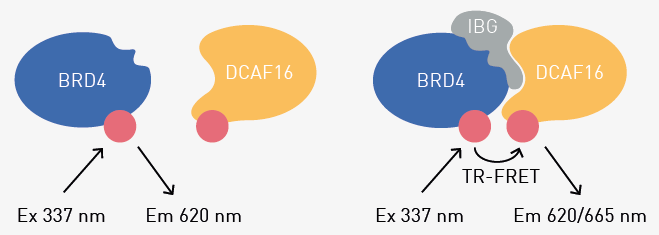
Fig. 1. Assay Principle of the TR-FRET-based molecular glue binding assay. Image Credit: Leif, F. et al., in partnership with ELRIG (UK) Ltd.
Materials & methods
- White 384-well plate
- Sulfo-Cy5 labeled DCAF16
- His-tagged BRD4 constructs
- Anti-His-europium donor (PerkinElmer)
- PHERAstar FS (BMG LABTECH)
Experimental procedure
TR-FRET-based molecular glue binding assays
Stock solutions of sulfo-Cy5-labeled DCAF16, His-BRD4, and anti-His-europium donor were prepared in TR-FRET assay buffer (50 mM HEPES pH 7.5, 100 mM NaCl, 1 mM TCEP, 0.05 % Tween-20).
Two types of TR-FRET assays were performed: titration of IBGs into a mix of BRD4 and Cy5-DCAF16 (complex-formation assay) and titration of sulfo-Cy5-labeled DCAF16 into BRD4 or BRD4 + IBG (complex-stabilisation assay).
For the former, compounds were titrated 1:4 into 100 nM BRD4 and 100 nM Cy5-DCAF16 to a white 384-well plate to a final well volume of 16 μL. For the complex-stabilisation assay, sulfo-Cy5-labeled DCAF16 was titrated 1:4 in TR-FRET assay buffer.
Final concentrations of 200 nM for BRD4 constructs and one μM for IBG1 were used. Europium anti-His6 donor and DMSO concentrations were kept constant across the plate for both assay formats at 2 nM and 0.5 %, respectively. Plates were spun down at 50 g for one minute and covered and incubated at room temperature for 30 minutes. Afterward, plates were read on the PHERAstar FS.
Instrument settings. Source: ELRIG (UK) Ltd.
| . |
. |
. |
| Optic settings |
TR-FRET, endpoint |
| Filters |
Ex TR
Em1: 665-10
Em2: 620-10 |
| General settings |
Integration time |
70-400 μs |
| Number of flashes |
200 |
| Settling time |
0 |
Results & discussion
A first aim in the presented study was to characterize the possible interactions between DCAF16, BRD4, and the bivalent glue IBG1 in vitro. The formation of a ternary complex between IBG1, DCAF16, and BRD4Tandem, a BRD4 construct containing both bromodomains (BD1 and BD2) connected by the native linker, was observed using isothermal titration calorimetry (ITC; data not shown).
Similarly, the time-resolved fluorescence resonance energy transfer (TR-FRET) complex-formation assay showed that a ternary complex formed between DCAF16 and BRD4Tandem in a dose-dependent manner upon IBG1 titration (EC50 = 44 nM; Fig. 2a). Overall, IBG1 was more active than its monovalent precursor JQ1.
A complementary TR-FRET-based complex-stabilization assay confirmed an interaction upon titrating DCAF16 into BRD4Tandem in the presence of IBG1 (Kd = 712 nM; Fig. 2b). Unexpectedly, an intrinsic affinity of DCAF16 to BRD4Tandem in the absence of IBG1 using TR-FRET (Kd = one μM; Fig. 2b) was also observed.
No such intrinsic affinity was observed with isolated BRD4BD1 or BRD4BD2, indicating that both bromodomains are required for complex formation. Comparison of the ITC titrations for DCAF16 into unbound versus IBG1-bound BRD4Tandem revealed that IBG1 strengthens (Kd of 0.6 μM versus four μM; data not shown) and thermodynamically alters the BRD4–DCAF16 interaction.
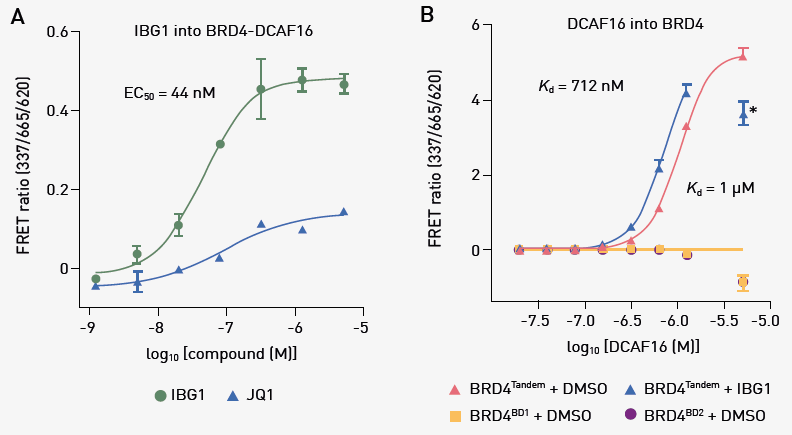
Fig. 2. A) TR-FRET ternary complex-formation assay. Europium-labeled anti-His bound to BRD4Tandem was incubated with equimolar Cy5-labeled DCAF16 and increasing concentration of IBG1 or JQ1. Mean ± SD. of n=3.
B) TR-FRET complex-stabilization assay. His-tagged BRD4Tandem or BRD4BD1 (200 nM) bound to anti-His-europium was incubated with increasing concentrations of Cy5-labeled DCAF16 in the presence or absence of 1 μM IGB1. Mean ± SD of n=2 independent experiments with two technical replicates. Image Credit: Leif, F. et al., in partnership with ELRIG (UK) Ltd.
With the mode of action of IBG1 confirmed, further compounds were synthesized to improve its molecular glue activity towards BRD4 and DCAF16. BRD4 degradation efficiencies of the new compound IBG3 exceeded those of IBG1, with IBG3 showing degradation in a low picomolar range (DC50 = 6.7 pM, data not shown). IBG3 also showed improved ‘gluing’ of the BRD4–DCAF16 complex by TR-FRET (EC50 = 32 nM; Fig. 3).
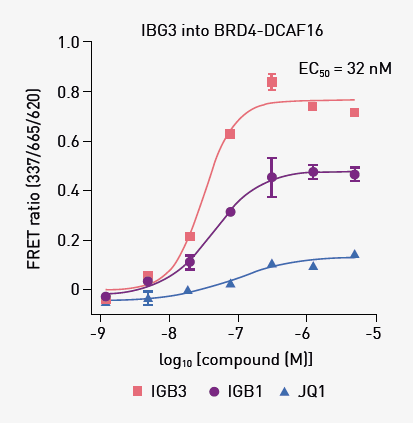
Fig. 3. TR-FRET ternary complex -formation assay. Anti-His-europium bound to BRD4Tandem was incubated with equimolar Cy5-labeled DCAF16 and increasing concentrations of IBG1, IBG3, or JQ1. Mean ± SD of n=3. Image Credit: Leif, F. et al., in partnership with ELRIG (UK) Ltd.
Similar to its parental compound, IBG3 was specific for BRD2 and BRD4 over BRD3, was selective for tandem bromodomains over isolated bromodomains, and mediated degradation via the same intramolecular glue mechanism, indicating that it is mediated by DCAF16.
Conclusion
Using the presented TR-FRET-based interaction assay, novel molecular glues targeting the BRD4-DCAF16 interaction could be characterized. These novel intramolecular bivalent glues follow a new interaction mechanism by binding to two domains of the target protein, thereby increasing its affinity to an E3 ligase.
This outlines a promising approach to pharmacologically utilize intrinsic interactions with diverse effector proteins and rewire cellular circuits for protein degradation and beyond.
References
Hsia, O., et al. (2024). Targeted protein degradation via intramolecular bivalent glues. Nature, [online] pp.1–8. https://doi.org/10.1038/s41586-024-07089-6.
About BMG LABECH
BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics, and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to ensure the highest quality of content and make it readily accessible to all. We will also strive to be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate, and collaborate on an open-access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025