This article and associated images are based on a poster originally authored by Sivakumar, M, Smith, E, Shumate, J, Scampavia, L, Spicer, T, Hernandez, D and Peters, C and presented at ELRIG Drug Discovery 2025 in affiliation with BMG LABTECH and UF Scripps Institute.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Ion channels are the second largest family of membrane proteins with at least 400 members. They are responsible for numerous normal physiological functions throughout the body, serving as the means to transport ions across the cell membrane.1
Channelopathies are a collection of genetic diseases that are caused by mutations of ion channel proteins. Therapies that target ion channels could be beneficial to a wide variety of diseases, including cancer and autoimmune diseases. Despite extensive drug discovery efforts, only a small number of ion channel family members have been successfully targeted for treatment. Herein, we describe an approach to expand the utilization of ion channel assays that will be beneficial in the search for additional therapies.1
Thallium-sensitive dye-based methods have made it possible to measure monovalent ion channels such as potassium channels. However, they typically employ kinetic imaging readers, which are not commonly found in laboratories. Here, a modified potassium channel assay is demonstrated that can be detected with the PHERAstar FSX with comparable results to kinetic modality readers.2
Assay principle
Cells expressing G protein-gated inwardly rectifying potassium channels (GIRK) were loaded with thallium as a surrogate for potassium and Thallos, a thallium-sensitive dye (Figure 1).
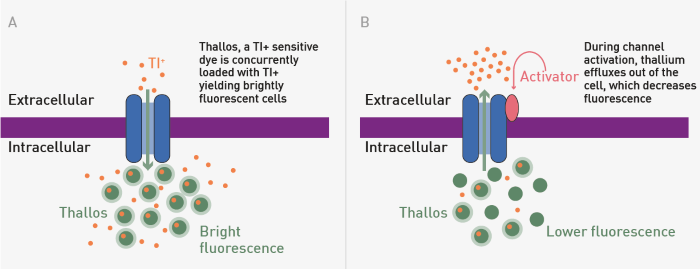
Fig 1. Thallium Flux Snapshot Assay Principle. A) The loading step B) Compound activation of a GIRK leads to efflux of thallium (Tl-) and decreased fluorescence signal. Image Credit: Image courtesy of Sivakumar, M et al., in partnership with ELRIG (UK) Ltd.
Materials & methods
- CHO-G12 cells and Brilliant Thallium Snapshot Flex kit (Ion Biosciences)
- 1536, black, TC-treated, microplates (#782078, Greiner)
- LOPAC 1280 Library (Sigma-Aldrich)
- PHERAstar FSX (BMG LABTECH)
- For the origin of other chemicals and reagents, please see Reference 2.
Experimental procedure
To seed CHO-G12 cells, the appropriate concentration was prepared to result in the indicated cell number, and 2.5 μL was dispensed to each well. Plates were centrifuged at 500 g for 5 min, followed by 24 h in an incubator (37 °C, 5 % CO2). 2.5 μL of Brilliant Thalium Snapshot loading solution was dispensed to all wells. Plates were centrifuged at 500 g for 5 min, followed by 30 min incubation at 37 °C and 5 % CO2.
A T0 read was performed using the settings indicated in the table below, followed by the addition of 30 nL of compound. Following the indicated incubation time, plates were read again (Tf). For detailed information on the method, please see Reference 2.
Instrument settings for cell assays. Source: ELRIG (UK) Ltd.
| |
|
|
| Optic settings |
Fluorescence Intensity, end point |
| Optic System |
Top Optic |
| Optic Module |
FI 485 520 |
| Gain |
400 |
| Focal height |
7.7 mm |
| General settings |
Flashes |
1 |
Results & discussion
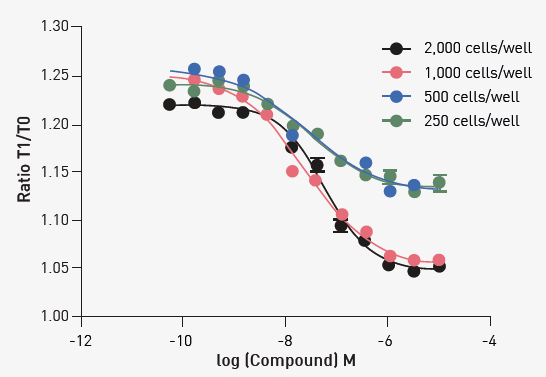
The optimization of the endpoint readout version of this assay began with understanding the effect of cell numbers. The initial assessments shown in Figure 2 were performed at the five-minute time point.
| |
2,000 c/w |
1,000 c/w |
500 c/w |
250 c/w |
| HillSlope |
-0.9565 |
-0.6394 |
-0.6381 |
-0.7347 |
| IC50 |
5.834e-008 |
2.435e-008 |
2.379e-008 |
3.169e-008 |
Fig 2. Optimization of assay cell number. The indicated number of cells was treated with varying concentrations of compound, and thallium efflux was determined by the Tf/T0 ratio produced by readings on the PHERAstar FSX after 5 minutes of incubation. Image Credit: Image courtesy of Sivakumar, M et al., in partnership with ELRIG (UK) Ltd.
The example data in Figure 2 indicates that 1000 cells/well exhibit good assay quality metrics. Extending the compound incubation time to 10 min showed additional improvement in assay quality (data not shown). Figure 3 shows the comparison between the PHERAstar FSX and an imaging reader under the optimized conditions.
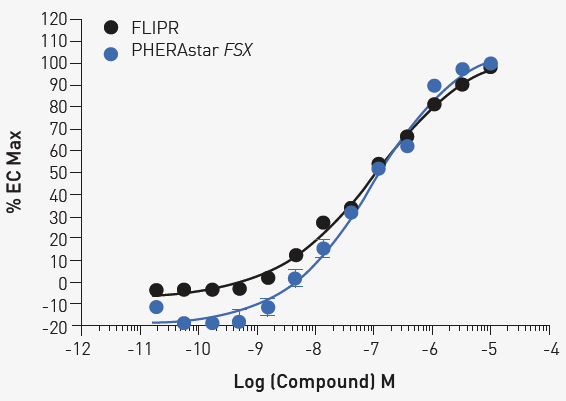
Fig 3. Concentration response under optimized conditions 1000 cells were treated with the indicated concentration of ML297 for 10 minutes. Comparable results are obtained with FLIPR or PHERAstar FSX. Image Credit: Image courtesy of Sivakumar, M et al., in partnership with ELRIG (UK) Ltd.
As further proof of concept, the adapted assay was employed in a pilot screen using the LOPAC 1280 compound library.
Each plate contained a quality control to determine plate Z’-factor, and only plates with a score of 0.5 or higher were deemed acceptable. Comparison of triplicate screening plates showed reproducibility (data not shown).
Following the pilot HTS, one compound exhibited confirmed dose responsiveness (Fig. 4). Zimelidine dihydrochloride is a known serotonin reuptake inhibitor.
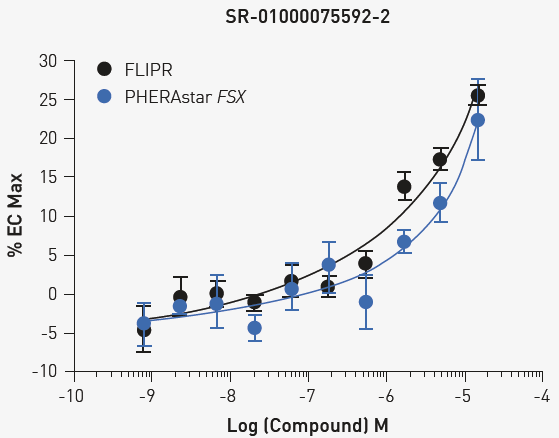
Fig 4. Concentration response for Zimelidine dihydrochloride. Cells were treated with the indicated concentrations and exhibited comparable responses on either reader. Image Credit: Image courtesy of Sivakumar, M et al., in partnership with ELRIG (UK) Ltd.
Conclusion
We show the adaptation of Snapshot technology for an endpoint potassium channel assay. The LOPAC pilot screening revealed no new hits, which is in line with the anticipated results. This simplified assay approach should prove useful for larger diversity library HTS campaigns, which are now enabled for a much greater number of screening labs that may not have access to kinetic imaging readers.
References
- Hutchings, C.J., Colussi, P. and Clark, T.G. (2018). Ion channels as therapeutic antibody targets. mAbs, 11(2), pp.265–296. DOI: 10.1080/19420862.2018.1548232. https://www.tandfonline.com/doi/full/10.1080/19420862.2018.1548232.
- Smith, E., et al. (2024). Protocol for kinetic mode potassium channel assays on common plate readers and microscopes. SLAS Discovery, 29(3), p.100148. DOI: 10.1016/j.slasd.2024.100148. https://linkinghub.elsevier.com/retrieve/pii/S2472555224000108.
About BMG LABECH
BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programs that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free of charge to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate, and collaborate on an open-access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Dec 12, 2025