This article is based on a poster originally authored by Darren Barrington-Light, Mircea Martiniuc, and Laercio Pires Fernandes Jr.
The Breath Biopsy® technique is emerging as a powerful method for developing novel non-invasive disease biomarkers and applications in precision medicine. Gas chromatography (GC) coupled with mass spectrometric (MS) detection remains the gold standard for the identification and quantification of the diverse breath metabolome.
Typical discovery workflows depend on untargeted analysis, which has significant limitations regarding robust compound identification (ID), translatability, and cross-study comparisons. Conversely, targeted analysis offers higher confidence data but is currently restricted to small panels of compounds, such as carbonyls.1
Large-scale targeted analysis, as described in the study presented here, will bridge the gap between the two approaches, enabling the development of the Breath Biopsy VOC Atlas®, Owlstone Medical’s expanding repository of breath-related volatile organic compounds (VOCs) and extensive metadata, thereby accelerating the advancement of breathomics applications.
Sample preparation
Sample collection was performed using Owlstone Medical’s ReCIVA® Breath Sampler in conjunction with the CASPER® Portable Air Supply to minimize ambient contamination, as previously outlined by Arulvasan et al.2
Approximately 1.25 L of breath was collected onto each sorbent tube. Deuterated internal standards (n = 36) were injected into all calibration standards and sample tubes.
Test method
Samples (single tube) were analyzed on a thermal desorption (TD) unit (Markes TD100xr) coupled to a GC-MS (Thermo Scientific Orbitrap Q Exactive mass spectrometer) system, following the Breath Biopsy OMNI® method.
The target list comprised of 200 compounds derived from breath, based on previously published work.2,3
A solution containing 36 deuterated internal standards (IS) was injected into all calibrators, check standards, and unknowns.
Data analysis
Processing methods, view settings, and report templates were created and executed in Chromeleon CDS due to its compliance, audit trail functionality, and capability to control and operate high-resolution accurate mass (HRAM) instruments in a networked environment.
Network architecture
Following this method, approximately 96 MB of data was generated per sample, with each full sequence producing around 6 GB.
Annually, data acquisition across Owlstone Medical has reached 5 to 6.6 TB, reflecting the substantial volume of HRAM data generated through routine operations.
The management of instruments and data handling was streamlined by utilizing Chromeleon CDS, version 7.3.2 MUb, deployed as a lab-wide enterprise solution within a cloud environment.
This scalable, centralized infrastructure ensured efficient data processing, secure storage, and enhanced accessibility to support scientific workflows.
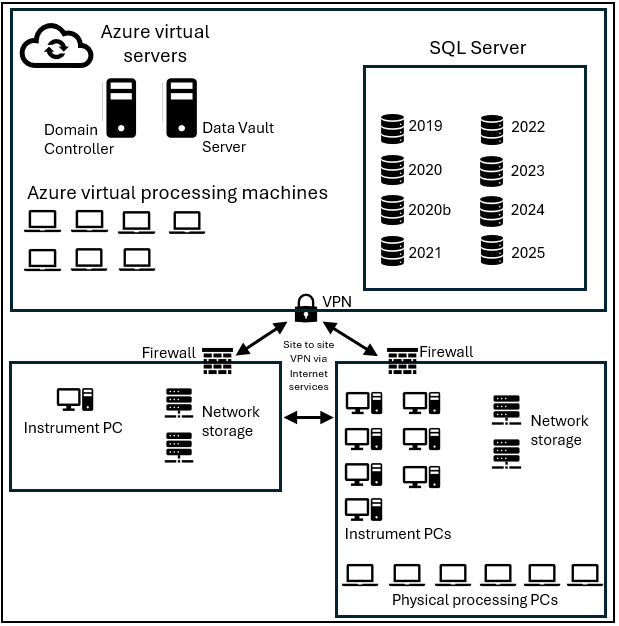
Figure 1. Cloud-based network allows users to control nine instruments and perform data analysis remotely with seamlessly integrated data vaults, instrument PCs and data processing virtual machines. Image Credit: Owlstone Medical Ltd
Integrity System Suitability Testing (SST) with Intelligent Run Control (IRC)
Thermal desorption analysis is destructive; therefore, known instrument and sample injection failure modes (e.g., failed sorbent tube pressure test) were implemented to prevent the analysis of samples expected to be unsuccessful.
In cases of more severe failures, acquisition halts, and relevant users were notified via email, facilitating rapid resolution.
Patient-derived samples generate vital data and are often irreplaceable, making reinjection impractical or impossible. Consequently, proactive management of out-of-specification (OOS) conditions is essential.
To preserve sample integrity and avoid invalid analyses, the system was configured to stop runs when OOS conditions are detected, either within the system itself or in preceding injections.
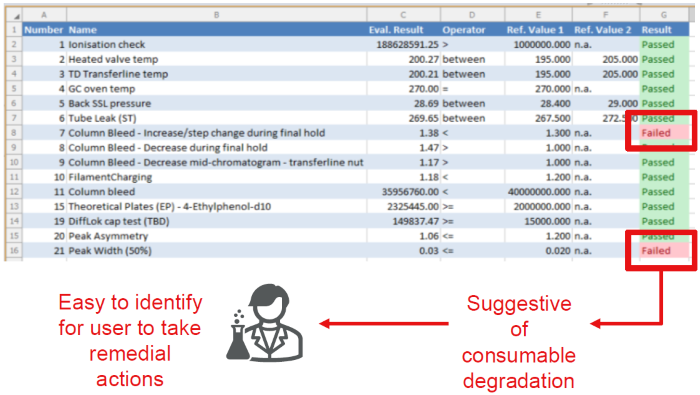
Figure 2. Interactive Results showing SSTs per injection with color-coded pass/fail criteria allow the analyst to assess the viability of the sample and sequence. Image Credit: Owlstone Medical Ltd
Composite scoring
The identity of each deuterated and target peak was confirmed using Composite Scoring, achieving high-confidence (Tier 1) identifications.4
This process combines mass accuracy (≤ 5 ppm), fragment ion coelution (≤ 0.01 min), and ion ratios (20% window) in comparison to authentic standards analyzed in each analytical sequence.
Furthermore, in-house HRAM libraries containing over 1,000 entries can be searched within the software to assist in the identification of compounds not present in the target list.
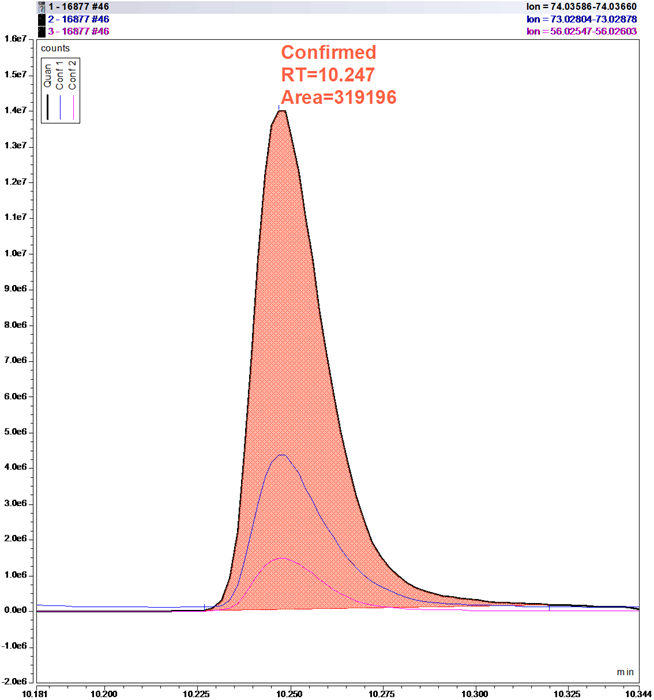
Figure 3. Target compound 2-Methylbutanoic acid in breath sample with “Confirmed” Composite Scoring result. Image Credit: Owlstone Medical Ltd
Automatic Gain Control (AGC)
IS correction and compound-specific linear regression with weighting (typically 1/Amount2) enabled direct quantitation of each target compound over linear dynamic ranges between 20 and 4,000.
The quantitation ranges are established to cover or exceed previously measured breath concentrations, reducing the requirement for reanalysis of out-of-range samples.
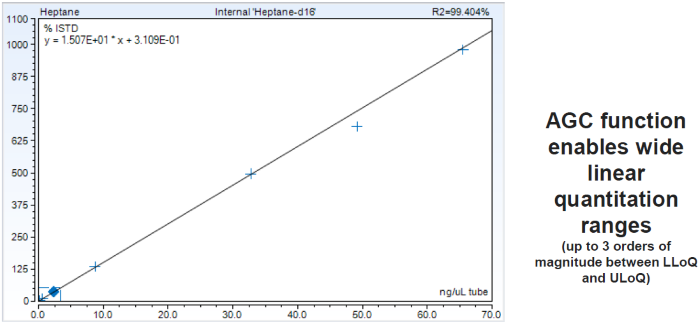
Figure 4. Calibration curve for heptane showing breath sample with amount within calibration range (0.05-65 ng). Image Credit: Owlstone Medical Ltd
View settings
Integrated tools for dataset tabulation and visualization facilitated the creation of customizable, sequence-wide plots and tables with interactive elements to enhance data analysis.
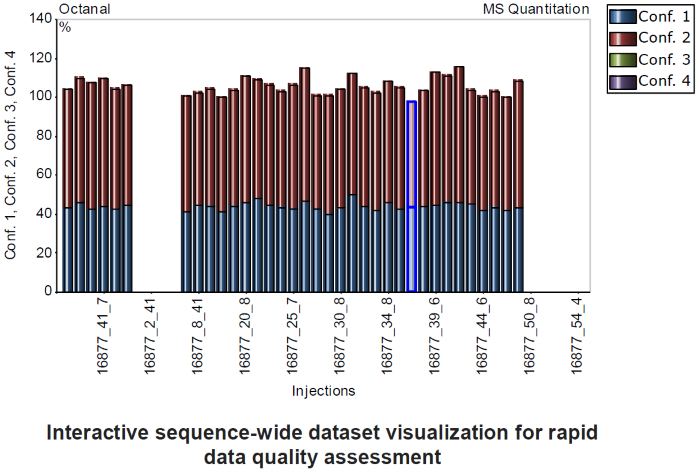
Figure 5. Interactive Chart showing ion ratios for a target compound throughout a sequence, with check standards (used as reference) on the left. Image Credit: Owlstone Medical Ltd
Real-world results
A method validation and verification study was conducted using two cohorts of breath samples. The complete analytical workflow was applied, including all elements of intermediate precision, along with significant instrument maintenance between the sample cohorts. Precision across the entire workflow was rigorously assessed.
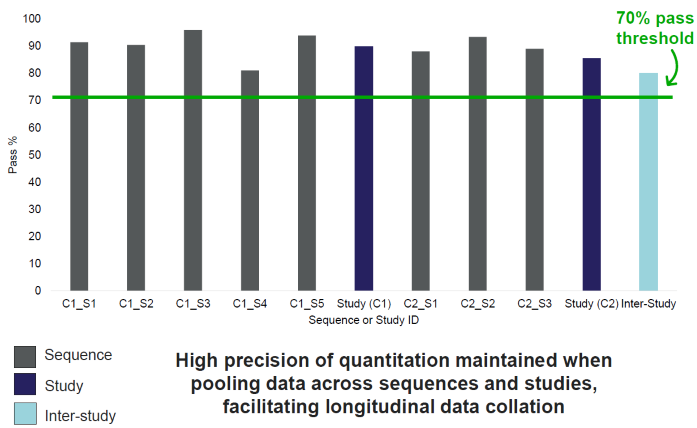
Figure 6. Proportion of target compounds (n=200) with check standard (n=6-7 per sequence) precision below 20 % RSD threshold within individual sequences, studies and between studies. C = Cohort, S = Sequence. Internal threshold of 70 % (≥140 compounds) applied. Image Credit: Owlstone Medical Ltd
Conclusions
An analytical workflow was developed entirely within Chromeleon software, encompassing the following:
- Enterprise-wide deployment in a cloud environment, providing scalable storage, centralized instrument control, and remote data access;
- Fully integrated, traceable, and compliant method for data acquisition, analysis, and reporting;
- Robust compound identification via composite scoring and ion ratio confirmation against reference standards, which supports improved data quality, high analytical confidence, and extensive compound coverage;
- Automated SST/IRC designed to prevent loss of vital patient samples by stopping acquisition in the event of instrument issues, while guaranteeing key method parameters stay within validated acceptance criteria;
- Interactive visualization and data review tools that facilitate efficient curation and guarantee the stability and reproducibility of quantitative results across sequences.
The output of this work was a fully integrated, compliance-ready quantitation workflow for the targeted analysis of breath biomarkers.
References and further reading
- Xie, Z., et al. (2024). Detection of COVID-19 by quantitative analysis of carbonyl compounds in exhaled breath. Scientific Reports, (online) 14(1). https://doi.org/10.1038/s41598-024-61735-7.
- Wisenave Arulvasan, et al. (2024). High-quality identification of volatile organic compounds (VOCs) originating from breath. Metabolomics, 20(5). https://doi.org/10.1007/s11306-024-02163-6.
- Wisenave Arulvasan, et al. (2025). Optimized breath analysis: customized analytical methods and enhanced workflow for broader detection of VOCs. Metabolomics, 21(1). https://doi.org/10.1007/s11306-024-02218-8.
- Schrimpe-Rutledge, A.C., et al. (2016). Untargeted metabolomics strategies – Challenges and Emerging Directions. Journal of the American Society for Mass Spectrometry, (online) 27(12), pp.1897–1905.https://doi.org/10.1007/s13361-016-1469-y.
Acknowledgments
Produced form materials originally authored by Darren Barrington-Light and Laercio Pires Fernandes Jr from Thermo Fisher Scientific, and Mircea Martiniuc from Owlstone Medical Ltd.
About Owlstone Medical Ltd
Owlstone Medical is developing a breathalyzer with a focus on non-invasive diagnostics for cancer, inflammatory disease and infectious disease, the company aims to save 100,000 lives and $1.5 B in healthcare costs.
The company’s Breath Biopsy® platform has introduced a new diagnostic modality making it possible to discover novel non-invasive biomarkers in breath using a platform with the potential to transition to point-of-care. The award winning ReCIVA Breath Sampler ensures reliable collection of breath samples.
Breath Biopsy is supporting research into early detection and precision medicine with applications in cancer and a wide range of other medical conditions. Highly sensitive and selective, these tests allow for early diagnosis when treatments are more effective and more lives can be saved.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Sep 16, 2025