New concepts of infectious disease are evolving with the realization that pathogens are key players in the development of progressive chronic diseases that originally were not thought to be infectious.
Infection is well-known to be associated with numerous neurological diseases for which pathologic effects have been well documented (Johnson, 1996). What has remained unclear, however, has been the role of infection as a causative or risk factor in the development of chronic neurodegenerative diseases.
In this regard, numerous studies over the past ~25 years have investigated whether there is an association between various infectious agents and Alzheimer’s disease, the most prevalent neurodegenerative condition accounting for dementia in the elderly.
Of the pathogens being considered in sporadic late-onset Alzheimer’s disease, Herpes Simplex Virus 1 (HSV-1) (Itzhaki et al, 1997), Borrelia species (Miklossy, 1993), and Chlamydia pneumoniae (Balin et al, 1998; Gerard et al, 2006) have garnered significant attention.
Work from other laboratories on systemic infectious disease (Kamer et al 2008, Cunningham et al, 2005) has also led to further interest in the role that infection may play in contributing to the neurodegenerative process in older populations.
Data from these investigations are intriguing, and have led to a renewed interest in investigating the role(s) of pathogens in the etiology of sporadic late-onset Alzheimer’s disease.
Furthermore, there is renewed interest in challenging long-held hypotheses in the Alzheimer’s research arena as investigations are uncovering more novel features of the amyloid protein as well as the inflammatory response associated with this disease.
The possibility of an infectious etiology of several chronic diseases, including Alzheimer’s disease (AD), has long been debated. Alzheimer, himself, over 100 years ago studied Treponema pallidum, the causative agent of syphilis, a spirochete later associated with dementia (Noguchi and Moore, 1913).
Sporadic late-onset Alzheimer’s disease, accounting for ~95-98% of all cases of Alzheimer’s disease, is thought to arise due to a multi-factorial interplay between genetic and environmental factors.
Speculation as to which environmental factors may have a great impact on the pathogenesis of this disease has resulted in studies of infectious disease.
This is a rational approach as different types of infections have been associated with dementing illnesses, including infection with Treponema pallidum, mentioned previously, as well as Cryptococcus neoformans (Hoffman et al, 2009), measles virus (Frings et al, 2002), and HIV (Zhou et al, 2010).
Early studies of infection directly related to Alzheimer’s disease attempted to correlate viral infection with late-onset disease (Pogo et al, 1987). Of the viruses considered were: Herpes Simplex 1 and 2, cytomegalo virus, measles virus, poliovirus, adenoviruses, hepatitis B virus, and the influenza A and B viruses. There was no association with disease determined for these viruses.
Later and more recent studies have found evidence for direct brain infection in AD with HSV1 (Itzhaki et al, 1997), Borrelia burgdorferi (Miklossy, 1993), and Chlamydia pneumoniae (Balin et al, 1998, Gerard et al, 2006).
There are some reports that even systemic infections may correlate to increased incidence of AD and infection with Helicobacter pylori, the agent of gastric ulcers and Porphyromonas gingivalis, an agent of periodontitis, have been studied in late-onset disease (see Honjo et al, 2009, Kim et al, 2007 for review).
Given these reports and the need to identify and understand causative factors for sporadic late-onset AD, much inquiry is needed to determine the mechanisms by which these different infections can initiate and participate in the pathogenesis of AD.
Interestingly, when one considers factors that may drive the accumulation of amyloid and tau in AD, infectious triggers are some of the most significant and logical choices; in particular, the organisms likely to be involved in AD are those that can evade host defenses, gain entry to specific selectively vulnerable regions of the brain, and establish chronic/persistent and/or latent infection. Upon considering the other risk factors found in AD, infection may be the central hub connecting these factors.
Currently, evidence from research on Chlamydia pneumoniae, Herpes Simplex Virus 1, and Borrelia burgdorferi in the AD brain, links numerous risk factors with infection to the pathogenesis of AD.
Linkage has been identified to risk factors including ApoEe4 expression, chronic neuroinflammation, autoimmune mechanisms, oxidative and mitochondrial damage, cardiovascular factors, diabetes with insulin resistance, trauma to the blood brain barrier and selectively vulnerable brain insult (for reviews see Journal of Alzheimer’s Disease, Vol. 13, #4, May 2008, pgs 357-463).
Thus, infection actually may be the overarching “unifying hypothesis” for sporadic late-onset AD, rather than other more mainstream hypotheses.
Alzheimer's Disease and Infection Research | PCOM
How much evidence is there to date that pathogens could be involved in the pathogenesis of Alzheimer’s?
If you search through pubmed or medline you will see that hundreds of peer-reviewed original research articles are indexed on infection in Alzheimer’s disease as you can see in this table.
|
Search
|
Query Pubmed search performed as of 12/2/2016
|
Items found
|
|
#9
|
Search (pathogen and Alzheimer's disease)
|
181
|
|
#8
|
Search ((((infection and Alzheimer's disease)) NOT Reviews)) AND parasite
|
10
|
|
#7
|
Search ((((infection and Alzheimer's disease)) NOT Reviews)) AND fungus
|
6
|
|
#6
|
Search ((((infection and Alzheimer's disease)) NOT Reviews)) AND Fungal
|
104
|
|
#5
|
Search ((((infection and Alzheimer's disease)) NOT Reviews)) AND Bacteria
|
188
|
|
#3
|
Search ((((infection and Alzheimer's disease)) NOT Reviews)) AND viruses
|
143
|
|
#2
|
Search ((infection and Alzheimer's disease)) NOT Reviews
|
702
|
|
#1
|
Search infection and Alzheimer's disease
|
1095
|
Which pathogens have you focused on in your research and why?
We have been studying infection within the AD central nervous system by Chlamydophila (Chlamydia) pneumoniae since the late 1990s. We believe that this unique intracellular bacterial organism plays a role in causing or contributing to late-onset sporadic Alzheimer’s disease (see Balin et al., 2008 – Journal of Alzheimer Disease for review).
In our first report of an association of C. pneumoniae with AD, we demonstrated by polymerase chain reaction (PCR) that the DNA of the organism was present in 90% of postmortem brain samples examined from sporadic AD (Balin et al., 1998).
A subsequent report from 2006 by us (Gerard et al) confirmed this finding in a different set of AD brains. As compared to the results in AD tissues, only 5% of postmortem brain samples from age-matched, non-AD, control individuals contained DNA from C. pneumoniae.
In the original and subsequent study, PCR was conducted using highly specific and sensitive probes for sequences of C. pneumoniae chromosomal DNA (Schumacher et al., 1999). Areas of the brain demonstrating significant neuropathology (eg, temporal cortices, hippocampus, parietal cortex, pre-frontal cortex) as well as areas less often demonstrating AD pathology (eg, cerebellum) were sampled by PCR.
Positive samples were obtained from at least one area demonstrating neuropathology, and in four cases, from the cerebellum. Interestingly, in the latter four cases, severe neuropathology was observed throughout, while in the two AD brains that were PCR-negative, very mild pathology was observed (Balin et al., 1998).
We have examined the role that C. pneumoniae plays in “triggering” events resulting in AD pathology following in vitro and in vivo (Little et al., 2004) infection with this organism. In vitro infections of the human cell lines, THP-1 monocytes and human brain microvascular endothelial cells (HBMECs), were designed to determine whether infection with C. pneumoniae influences the production and/or processing of cellular proteins found to be important in AD pathology.
Data from these studies suggested that C. pneumoniae infection of both cell types resulted in an increase in Ab 1-40 and Ab 1-42 immunoreactive peptides over the course of a 72hr infection.
The increase in immunoreactive fragments was determined following analysis of whole cell lysates. The processing of amyloid by these cell types, in addition to their activation to promote transmigration of peripheral monocytes through an in vitro blood brain barrier which we also demonstrated (MacIntyre et al., 2003), suggests that angiopathy and neuroinflammation could arise following local amyloid production and processing in the brain.
The influx of infected monocytes into the brain with ultimate production and processing of b-amyloid may provide a nidus of amyloid deposition that could promote further damage and/or “seeding” of further plaque development.
Entry of infected monocytes into the brain also may result in infection of resident microglia, astroglia, and neurons. Alternatively, a second pathway of infection through the olfactory neuroepithelia may lead to more direct infection of neurons in the specific limbic system regions of the brain such as the entorhinal cortex and hippocampal formation.
We believe that this olfactory route of infection actually may be the most important for the initiation of damage to the areas of the brain (ie, lateral entorhinal cortex and hippocampus) that are first involved with pathology in Alzheimer’s disease.
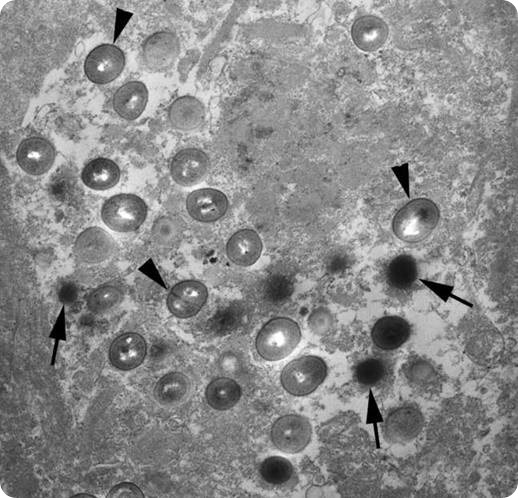
Electron micrograph of a section of brain tissue from an Alzheimer's brain showing the chlamydial bodies inside a glial cell in the brain. The arrowheads point to what are termed reticulate bodies and the arrows point to elementary bodies - both types of bodies are different life forms of Chlamydia pneumoniae. . Image credit: The Balin Laboratory.
Why is this a controversial area?
This avenue of research remains controversial for a number of reasons.
- The “amyloid cascade hypothesis” has remained in favor for ~the past 30 years and the field is still fixated on this area even after ~430+ clinical trial failures with most of these trials based on this hypothesis.
- Different organisms have been identified to be associated with AD – many having been found in brain tissues, and others found systemically that could lead to neurological change – as there could quite well be a polymicrobial influence on this neurodegenerative condition, there may be multiple mechanisms behind manifestation of disease, Of the naysayers, they believe that not enough evidence has been found to change the existing paradigm for causation of AD.
- The fact that many of the infections that have been found in the disease are of a persistent, chronic and/or latent nature makes it difficult to assign starting points for when a person becomes infected and how this could result years later in Alzheimer’s disease.
- The lack of acceptance or understanding that a number of organisms, including Chlamydia pneumoniae and HSV1 can enter the brain by bypassing the blood brain barrier (ie, using our sense of smell) has resulted in a lack of appreciation for this process to initiate late-onset AD. Not enough neuropathologists understand how these types of organisms can promote inflammation and the generation of amyloid and/or tau pathology even though we and many others have demonstrated this experimentally after finding these organisms in human AD brains.
So, in general the vast majority of the AD research field has focused and continues to focus on the pathology that occurs in the disease and not on the initiating triggers for this pathology.
What further research is needed at this stage?
The further work needed at this stage is better understanding of mechanisms or pathways that are influenced by infection that lead to neuroinflammation and nerve cell damage that correlates to that observed in AD brains.
This would hold for both infections that actually enter and are found in the brain, as well as those that occur outside the brain but could influence neurological functioning.
Also, clinical trials to obtain biomarkers based on infections as well as those for treatment using antivirals, antibacterials, and anti-inflammatory drugs are required to establish how treating for infection can potentially influence onset and/or progression of disease following early diagnosis.
In addition, much more research is needed that involves the microbiome of the gut and pulmonary systems with regards to how these microbiomes can influence neurological health either directly or indirectly.
Furthermore, research on the anti-infective properties of amyloid must continue as this provides a rationale for why infection would be involved with late-onset Alzheimer’s disease and how amyloid generation and accumulation would be triggered to combat infection in the AD brain (Kumar et al, 2016; Spitzer et al, 2016).
In the future do you think it will be possible to develop diagnostic tools to determine who may be at risk for Alzheimer’s?
Yes, the current diagnostic tools of looking for biomarkers in blood and CSF have focused on differences in pathological markers such as those for beta amyloid and tau proteins, but also for lipids, inflammatory proteins, and antibodies to ingredients from cellular components from the nervous system.
Differences between those with AD and normals are trying to be correlated. We believe that eventually other biomarkers, especially those for infection such as antibodies to infecting agents and affiliated immune responses would be more realistic and specific for AD.
Also, using smell testing along with analysis of infection could be helpful for determining how the changes in sense of smell (which do occur early in AD) would indicate that infection is onboard in this region of the brain. This could result in very specific therapeutic regimens (even those delivered intranasally) that could eradicate or limit the effects of infection in this population.
Is it likely that Alzheimer’s could be a treatable condition one day?
Yes, but we must identify the triggers of the disease first and focus on eradicating the triggering mechanisms, in this case infections, by using antibiotics, antivirals or other anti-infectives.
In addition, we may be able to use anti-inflammatory therapies or immune modulating therapies that may minimize neuroinflammation.
Possibly, anti-amyloid therapies could then be used in conjunction to minimize the accumulation of amyloid plaques. However, current use of anti-amyloid therapies without eliminating the triggering insults have not been successful.
Do you think it will ever be possible to prevent the condition entirely? Could vaccines be a possibility?
Yes, with acceptance that infections along with other risk factors are involved with this disease, we could actually prevent the late-onset form of Alzheimer’s disease entirely.
Other risk factors that need to be better controlled for would include: atherosclerosis, diabetes, head trauma, systemic infection and respiratory problems including air pollution.
Vaccines could be a possibility, but those against certain infections, like Chlamydia pneumoniae and Herpes Simplex Virus 1 and may be even Lyme disease (Borrelia burgdorferi) would be required.
Vaccines against amyloid and other naturally occurring entities in the body are probably not going to be useful in this disease and, to date, have failed in clinical trials.
What are the main hurdles that need to be overcome in this field of research?
The main hurdles to be overcome at this point in time is the myopia of the Alzheimer’s research arena which has focused on an enormous amount research with regards to amyloid and tau proteins which comprise the main pathologies in this disease.
With over 430 clinical trial failures based principally on pathological entities, researchers, clinicians and the pharmaceutical companies must seriously start considering other research such as that on infection that correlates not only to the pathology observed, but also to the pathways damaged early in the disease process and the resultant consequences such as neuroinflammation that lead to accumulating and progressive damage that is observed in late-onset disease.
Where can readers find more information?
Readers can find more information on the web in a number of ways.
1. Googling my name and/or the role of infection in Alzheimer’s disease.
2. Going to our website which is www.pcom.edu and searching under research for Dr. Brian Balin.
3. Emailing me directly at: [email protected].
4. Searching through the National Library of Medicine at www.ncbi.nlm.nih.gov/pubmed on infection in Alzheimer’s disease.
About Dr Brian J. Balin
 Dr. Brian J. Balin is the Chairman of the Department of Bio-Medical Sciences as well as a tenured full Professor of Experimental Neuropathology at Philadelphia College of Osteopathic Medicine.
Dr. Brian J. Balin is the Chairman of the Department of Bio-Medical Sciences as well as a tenured full Professor of Experimental Neuropathology at Philadelphia College of Osteopathic Medicine.
In addition, Dr. Balin is the Director of the Center for Chronic Disorders of Aging, an Osteopathic Heritage Foundation endowed center, as well as the co-director of the Adolph and Rose Levis Foundation Laboratory for Alzheimer’s Disease Research at PCOM.
Dr. Balin is an internationally recognized expert in the field of Alzheimer's Disease research.
His research interests include: Infection with Chlamydia pneumoniae as a trigger in the neuroinflammation and neurodegeneration in Alzheimer's Disease, effects of infection and inflammation on the blood brain barrier in cerebrovascular disease, modification of neuronal cytoskeletal proteins through phosphorylation and the action of transglutaminase, and pathogenesis of chronic disease associated with the aging process.
Dr. Balin has received numerous National Institutes of Health and private foundation grant awards for his research. He is highly published in peer-reviewed journals, and has written a number of chapters and reviews on the pathology of Alzheimer's disease, including reviews on the "Pathogen Hypothesis" of this disease (see live discussion section, www.alzforum.org).
In addition, he has presented and continues to present his work at major national and international scientific meetings including a number of international and world congresses on Alzheimer's disease and related dementias.
References:
- Balin B. J., Gerard H. C., Arking E. J., Appelt D. M., Branigan P. J., Abrams J. T., Whittum-Hudson J. A. and Hudson A. P. (1998). Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain, Med Microbiol Immunol (Berl) 187, 23-42.
- Cunningham C., Wilcockson D.C., Campion S., Lunnon K., and Perry V.H. (2005). Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neruodegeneration. J Neurosci 25, 9275-9284.
- Frings M., Blaeser I., Kastrup O. (2002). Adult-onset subacute sclerosing panencephalitis presenting as a degenerative dementia syndrome. J Neurol 249, 942-943.
- Gérard H. C., Dreses-Werringloer U., Wildt K.S. et al (2006). Chlamydophila (Chlamydia) pneumoniae in the Alzheimer's Brain. FEMS Immunol Med Microbiol 48, 355-66.
- Hoffmann M., Muniz J., Carroll E., and De Villasante J. (2009). Cryptococcal meningitis misdiagnosed as Alzheimer's disease: complete neurological and cognitive recovery with treatment. J Alzheimer’s Dis. 16, 517-520.
- Honjo K., van Reekum R., Verhoeff N.P. (2009). Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement 5, 348-60.
- Itzhaki R. F., Lin W. R., Shang D., Wilcock G. K., Faragher B., and Jamieson G. A. (1997). Herpes simplex virus type 1 in brain and risk of Alzheimer's disease [see comments], Lancet 349, 241-4.
- Johnson R.T. (1996). Emerging viral infections. Arch Neurol 53, 18022.
- Journal of Alzheimer’s Disease, Vol. 13, #4, May 2008, pgs 357-463
- Kamer A.R., Dasanayake A.P., Craig R.G., Glodzik-sobanska L., Bry M., and de Leon M.J. (2008). Alzheimer’s Disease and peripheral infections: The possible contribution from periodontal infections, model and hypothesis. J Alzheimer’s Dis. 13, 437-449.
- Kim J.M., Stewart R., Prince M., Kim S.W., Yang S.J., Shin I., and Yoon J.S. (2007). Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry 22, 850-855.
- Miklossy J. (1993). Alzheimer's disease--a spirochetosis?, Neuroreport 4, 841-8.
- Noguchi H., and Moore J.W. (1913). A demonstration of Treponema pallidum in the brain of general paralysis cases. J Exp Med 17, 232-238.
- Pogo B.G., Casals J., Elizan T.S. (1987). A study of viral genomes and antigens in brains of patients with
- Alzheimer’s disease. Brain 110, 907-15.
- Zhou L., Diefenbach E., Crossett B., Tran S.L., Ng t., Fizos H., Rau R., et al. (2010). First evidence of overlaps between HIV-associated dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia. Mol Neurodegener 5, 1-20.