As the COVID-19 pandemic continues to threaten global health, scientists have noted severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects males with greater frequency and severity and a higher mortality rate.
Prior research has found the spike protein to be capable of immune evasion and target receptor binding via its heavily glycosylated coat. This allows it to mediate the successful entry of the virus into the host cell.
Glycans Enhance ACE2 Affinity for Spike RBD
The researchers used molecular dynamics simulations (MDS) to reveal that the binding of glycans to the ACE2 receptor increases the spike protein affinity for it via interactions between the glycans and other glycans, glycans with proteins, as well as through hydrogen and hydrophobic bonds. They show that the ACE2 glycan at N322 and N546 interact with the glycans on the receptor-binding domain (RBD) of the spike protein at N165 and N343.
The glycans continue to display a high and unchanged affinity for anchoring molecules with a high negative charge, like the ACE2 protein, as well as to the S protein with its high polar charge. This might mean that spike RBD-ACE2 binding, as well as cell infection, are mediated by surface tethering as a result of such interactions and electrostatic forces.
Subsequently, they evaluated the effect of glycosylation of the ACE2 receptor on viral entry via the spike RBD in human umbilical vein endothelial cells (HUVECs) in culture, exploring a range of sugars. They confirmed that almost all sugar-treated cells had increased glycosylation, and enhanced S-RBD entry by eight times compared with those cells cultured in low-sugar media. Thus, the sugar-attached residues around the pocket at the top of the ACE2 molecule can enhance binding by the spike RBD.
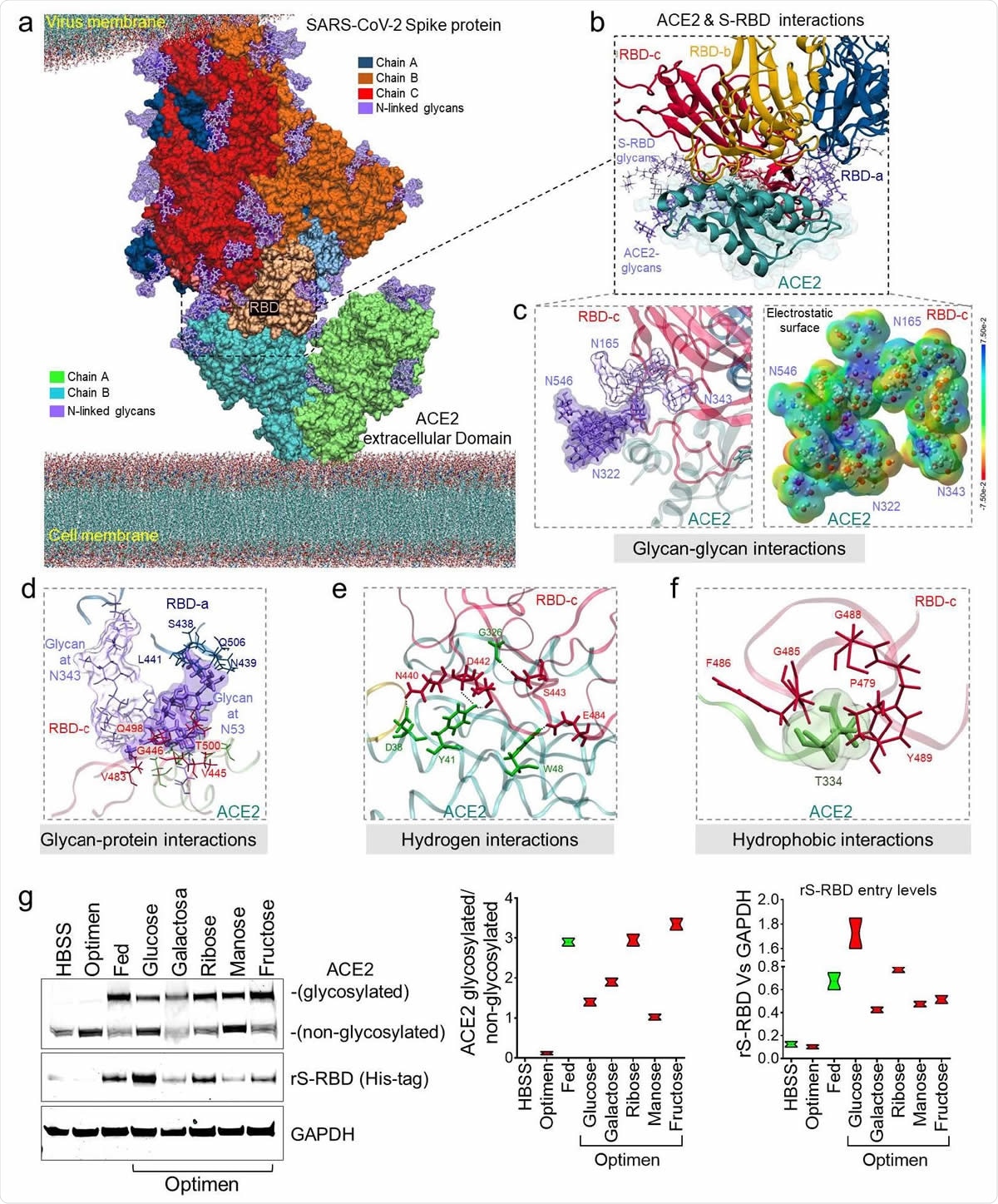
Molecular bases of glycosylated hACE2 and SARS-CoV-2 Spike protein complex. (a) 3D membrane surface representation of glycosylated ACE2 in complex with the SARS-CoV2 Spike protein. (b) Close up of the interacting environment between ACE2 and the S-RBD trimer. (c) The left panel demonstrate glycan-glycan interactions between ACE2 (dark purple surface) and S-RBDc (light purple surface). The right panel shows that glycan-glycan contacts do not affect their molecular electrostatic potentials (MEPs) properties. The energy scale ranging from -0.075 μa (red) to 0.075 μa (blue). (d) ACE2 glycan at N53 forms glycan-protein contact with residues on the S-RBDa and S-RBDc proteins. (e) The ACE2 glycosylation induce the formation of hydrogen bonds that engages the helix α1 in the binding with multiple residues on the S-RBDc. (f) Hydrophobic interactions occur between ACE2 at T334 and multiple residues on the S-RBDc. (g) Immunoblot showing expression of glycosylated and non-glycosylated human ACE2 in HUVECs treated with difference saccharides. Glucose-treated cells exhibited the greatest internalization of the recombinant S-RBD. Quantification of protein levels of three replicate experiments is shown. Student’s T-test, 2 tails. Bar graphs are presented as mean with error bars (±SD).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Estrogens Reduce ACE2 Glycosylation and Membrane Expression
Secondly, they looked at how estradiol and a dietary plant estrogen, S-equol, affected the structure and function of the ACE2 receptor. They found three regions of the receptor that are involved in virus binding, all of which are surrounded densely by glycans. These glycans interact with estradiol and S-equol, which stabilize the underlying helical structures via physical interactions. This binding reduces the free energy and interaction energy between the RBD and the ACE2 molecule.
When other estrogen molecules interact with the hydrophobic pocket surrounding the residue T334, the energy decreases by 12%, which could prevent RBD-ACE2 binding. Estrogens also interact with the glycan-protein interactions occurring between the ACE2 and RBD. These interactions between estrogens and various glycans reduce the molecular electrostatic potential and decrease the adhesion of S-RBD and ACE2.
Here, the estrogens act as ACE2 ligands as they can bind to the high-energy binding cavity at the top of the ACE2 molecule to stabilize its conformation and perhaps enhance its entry into the cell. Using the same HUVECs, they found that cells treated with estrogen showed decreased ACE2 on the membrane because estrogens increase the rate of internalization and breakdown of ACE2 via endocytosis. Thus, estrogens reduce ACE2 glycosylation and membrane expression in treated cells, confirmed by intratracheal administration of estrogens in male rats.
Estrogens Block SARS-CoV-2 Entry into Host Cell
The researchers also found that estrogens prevent ACE2-RBD binding because of the decline in free energy and increased stability of ACE2 following its interactions with estrogen molecules. When estradiol is involved, the RBD was observed to shift laterally, reducing the number of residues that come into contact with the receptor and abolishing contact at key glycosylated residues on the ACE2 receptor.
S-equol interacts with 145 residues on the receptor surface, while estradiol interacts with 66. Since ACE2 tends to bind to polar molecules and especially to electrophiles, the presence of low negative charge along with a few polar groups on the estrogen molecules increases their tendency to bind ACE2, thus hindering the residues that participate in S-RBD binding. Estradiol and S-equol reduced atomic contact by 80% and 65%, respectively, reducing the surface area on the receptor available for S-RBD binding.
In the HUVEC assay, they found that estrogens at high or low concentrations reduced spike RBD entry into the host cell by 90% or more, and this may be one way in which these hormones protect women and those with high phytoestrogen intake from severe COVID-19.
Estrogens Prevent Respiratory Infection by SARS-CoV-2
Using male mice, treated with intratracheal estrogen for 24 hours and exposed to the SARS-CoV-2 protein, the researchers found a markedly reduced affinity of ACE2 to the viral spike RBD on intratracheal administration. This resulted in increased concentrations of the viral protein on the surface of the lung cells, indicating a failure to internalize and, therefore, a block to viral infection.
Implications and Applications
The researchers point out that the increased glycosylation of the ACE2 receptor in a high-sugar environment caused increased S-RBD entry into the cell in vitro, which could partly explain why people with diabetes are at increased risk for severe disease and death in COVID-19. Secondly, the use of the predominant natural estrogen in humans and the dietary phytoestrogen helps uncover the mechanism of protection against the virus in women and those populations with a high intake of these plant estrogens.
Earlier studies have shown the similar protective effects of estrogens against other viral infections, including Ebola, hepatitis, and HIV. The lower estrogen levels in women following menopause could be one reason for the increased severity in this group of COVID-19 patients.
The researchers conclude: “We provide a molecular basis that helps elucidate the potential protective effect of estrogens in women infected by the SARS-CoV-2 virus which could inform the development of future therapeutic measures.”
These could include blocking antibodies designed along the lines of estrogens, estrogenic compounds themselves, and vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources