Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Symptoms of COVID-19 include mild to severe fever, cough, and headaches, though some people can have massive immune responses, respiratory failure, hospitalization, and even death. The risk factors for COVID-19 include older age, male sex, cardiovascular and kidney disease, obesity, and chronic obstructive pulmonary disease, among others.
It has been almost a year since the zoonotic entry of SARS-CoV-2 into the human population. To date, it has infected over 50 million people, causing over 1.25 million deaths across the world. The resulting pandemic has also induced widespread economic and societal disruption, making the invention of a vaccine a matter of utmost urgency. Leading vaccine candidates for SARS-CoV-2 use viral vectors to deliver the viral spike protein to the target cells. These vectors are encoded by mRNAs.
Recently, researchers from The Johns Hopkins University School of Medicine, Baltimore, MD, USA, and Capricor Therapeutics, Inc., Beverly Hills, CA, USA, described a new approach to SARS-CoV-2 vaccine development using exosomes to deliver mRNAs that encode the antigens from multiple structural proteins of the virus. Their study has been demonstrated on the preprint server bioRxiv*.
"In the present report, we established that formulations of purified exosomes, in vitro synthesized mRNAs, and polycationic lipids can mediate mRNA transport into human cells, and functional expression of mRNA-encoded protein products."
Exosome-mediated delivery of mRNAs that encode for SARS-CoV-2 structural proteins
The researchers purified the exosomes and loaded them with the mRNAs designed to express an artificial fusion protein – LSNME - that has parts of the viral spike, membrane, nucleocapsid, and envelope proteins also a functional form of the spike protein.
The resulting combination vaccine - LSNME/SW1 - was injected into 13-week-old C57BL/6J mice, and the humoral and cellular immune responses to the nucleocapsid and spike proteins of SARS-CoV-2 were assessed. Hematological and histological analysis was also carried out to look for possible adverse reactions in the vaccinated animals.
Immunized mice show CD4+ and CD8+ T-cell responses to SARS-CoV-2 proteins
The immunized mice developed CD4+ and CD8+ T cell responses to both the nucleocapsid protein and the spike protein of the SARS-CoV-2 virus. These responses were detected almost two months post-vaccination, as expected in the case of a durable response to vaccination. The researchers found that the CD4+ T cell response to the spike protein was associated with increased interferon-gamma expression. This indicates a Th1 response and a lesser induction of interleukin 4, a cytokine associated with Th2.
The vaccinated mice did not show any sign of altered growth, hypersensitivity at the injection site, white blood cell profile changes, or changes in the morphology of organs. Consistent with these findings, the team also detected moderate anti-nucleocapsid and anti-spike antibodies in the plasma of the vaccinated mice.
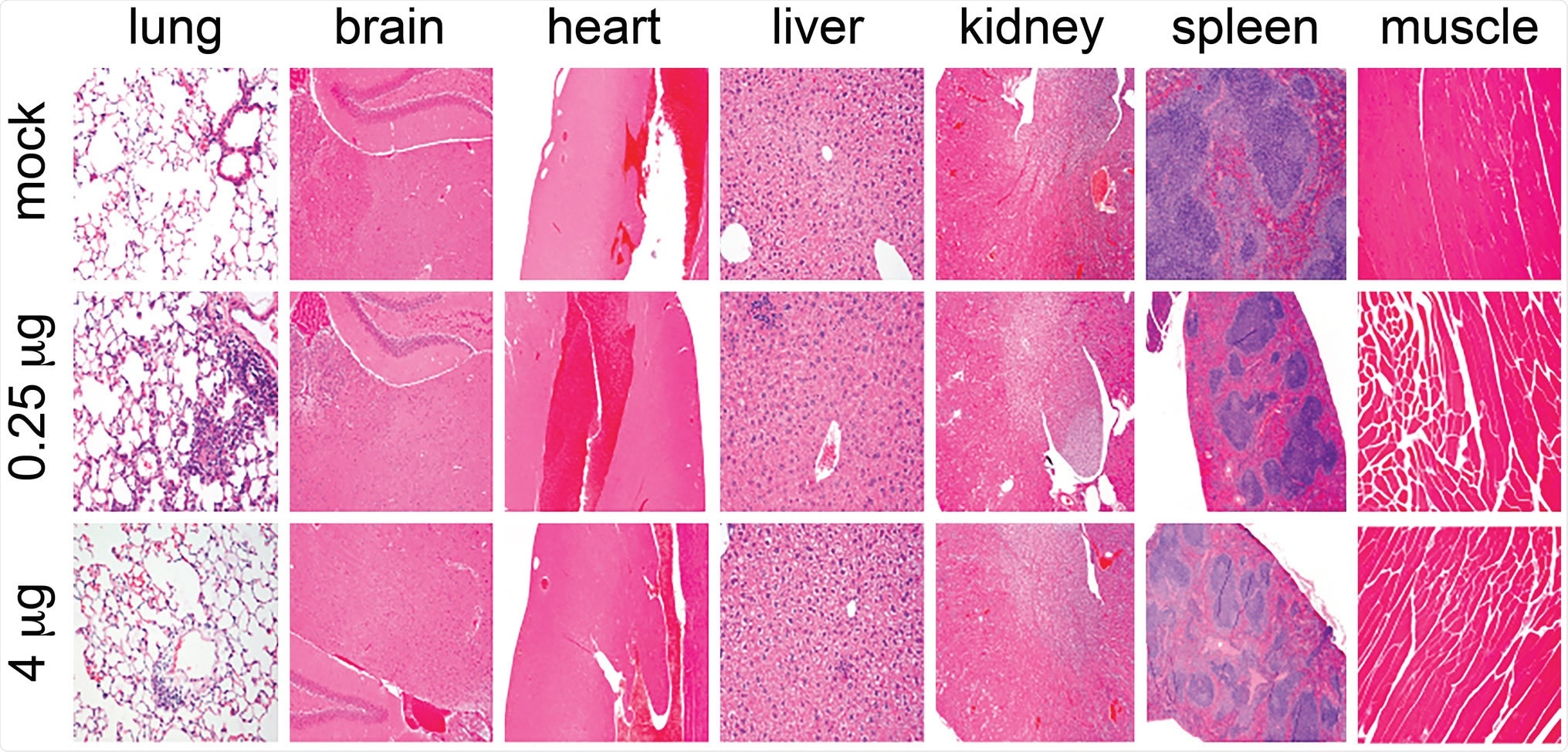
Absence of tissue pathology upon LSNME/SW1 vaccination. Representative micrographs from histological analysis (hematoxylin and eosin stain) of lung, brain, heart, liver, kidney, spleen, and muscle (side of injection) of animals from (upper row) control mice, (middle row) mice immunized with the lower dose of the LSNME/SW1 vaccine, and (lower row) mice immunized with the higher dose of the LSNME/SW1 vaccine.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Findings validate the use of exosomes to deliver mRNAs into target cells
In this study, the researchers established that purified exosomes can successfully carry mRNA into human cells for the functional expression of the proteins encoded by the mRNA. They first established this for a bioluminescent, fluorescent protein called Antares2 that acted as a reporter protein for determining the impact of different exosome-mRNA formulations on exosome-mediated mRNA delivery.
Overall, the study's findings validate the use of exosomes in the delivery of functional mRNAs into in vitro and in vivo target cells. More specifically, the results prove that the LSNME/SW1 vaccine can induce broad immunity to various types of SARS-CoV-2 proteins.
According to the authors, the successful development of the LSNME/SW1 vaccine in the future will depend on follow-up studies in bigger animal models at doses comparable to similar mRNA vaccines that successfully demonstrate a combination of safe and balanced immune reactions as well as protection against the SARS-CoV-2 virus.
"The successful use of exosomes to deliver Antares2 mRNA opens the door to follow-on studies aimed at optimizing exosome-RNA formulation conditions, as well as for characterizing the time-dependence of Antares2 expression, the biodistribution of exosome-mediated RNA expression, injection site effects, and exosome-mediated tissue."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Exosome-Mediated mRNA Delivery For SARS-CoV-2 Vaccination Shang-Jui Tsai, Chenxu Guo, Nadia A Atai, Stephen J Gould bioRxiv 2020.11.06.371419; doi: https://doi.org/10.1101/2020.11.06.371419, https://www.biorxiv.org/content/10.1101/2020.11.06.371419v1
- Peer reviewed and published scientific report.
Tsai, Shang Jui, Nadia A. Atai, Mafalda Cacciottolo, Justin Nice, Arjang Salehi, Chenxu Guo, Alanna Sedgwick, Saravana Kanagavelu, and Stephen J. Gould. 2021. “Exosome-Mediated MRNA Delivery in Vivo Is Safe and Can Be Used to Induce SARS-CoV-2 Immunity.” Journal of Biological Chemistry 297 (5): 101266. https://doi.org/10.1016/j.jbc.2021.101266. https://www.jbc.org/article/S0021-9258(21)01069-3/fulltext.