A landmark study shows that bat viruses closely related to SARS-CoV-1 and SARS-CoV-2 emerged far from outbreak sites, pointing to the wildlife trade, not natural bat movement, as the most plausible route into human populations.
 Study: The recency and geographical origins of the bat viruses ancestral to SARS-CoV and SARS-CoV-2. Image Credit: All-stock-photos / Shutterstock
Study: The recency and geographical origins of the bat viruses ancestral to SARS-CoV and SARS-CoV-2. Image Credit: All-stock-photos / Shutterstock
A new study by an international team of researchers led by the University of California, San Diego School of Medicine highlights a vital role of wildlife trade in triggering the emergence of coronavirus disease 2019 (COVID-19) in humans. The findings are published in the journal Cell.
Background
Horseshoe bats (Rhinolophus spp.) are the primary hosts of sarbecoviruses, viruses that typically infect bats harmlessly but have spilled over into humans through intermediate hosts. Severe acute respiratory syndrome (SARS)-related coronaviruses, including SARS-CoV-1 (causative pathogen of the SARS pandemic in 2002) and SARS-CoV-2 (causative pathogen of the COVID-19 pandemic in 2019), are viral species within the sarbecovirus subgenus.
Although existing evidence suggests that ancestors of SARS-CoV-1 and SARS-CoV-2 circulating in horseshoe bats gave rise to the human pandemics, it remains largely uncertain how these viruses reached the specific locations where spillover occurred, and whether wildlife trade, rather than natural bat dispersal, facilitated their spread.
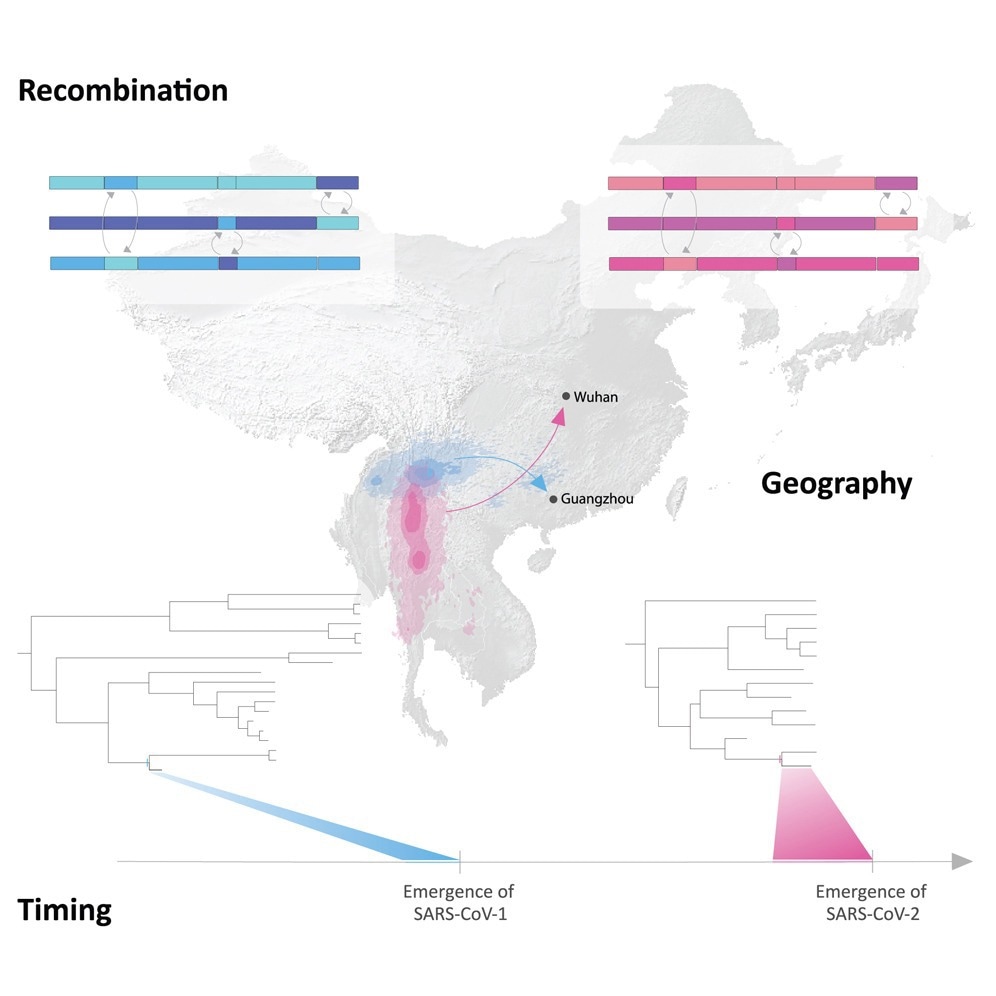
To explore these questions, researchers from 20 institutions across the U.S., Europe, and Asia analyzed the evolutionary relationships of SARS-CoV-1 and SARS-CoV-2 using advanced genetic recombination detection and Bayesian phylogeographic modeling. By accounting for genetic recombination and substitution saturation over time, they reconstructed the viruses’ evolutionary history across Asia before they emerged in humans.
Graphical abstract
Study Findings
The analysis revealed that sarbecoviruses related to SARS-CoV-1 and SARS-CoV-2 have circulated in bat populations across Western China and Southeast Asia for millennia. Using a “prisoner of war” (PoW) molecular clock model to correct for substitution saturation, the team found these viruses moved at rates comparable to their bat hosts’ foraging ranges (~2–3 km nightly).
However, the closest-inferred bat virus ancestors of SARS-CoV-1 and SARS-CoV-2 emerged less than a decade before each pandemic—SARS-CoV-1’s ancestor in southwestern China (1996–2001) and SARS-CoV-2’s in northern Laos or Yunnan, China (2007–2014). These viruses would need to travel over 1,000 kilometers to reach their human emergence sites in Guangdong (SARS-CoV-1) and Wuhan (SARS-CoV-2)—a journey implausible through natural bat dispersal alone.
“We show that the original SARS-CoV-1 was circulating in Western China—just one to two years before the emergence of SARS in Guangdong Province, South Central China, and SARS-CoV-2 in Western China or Northern Laos—just five to seven years before the emergence of COVID-19 in Wuhan,” said Jonathan E. Pekar, Ph.D., lead author and postdoctoral researcher at the University of Edinburgh.
The study also tested hypothetical unsampled viral diversity, confirming that their findings remained robust even if closer relatives existed in under-sampled regions.
Wildlife Trade as the Missing Link
The researchers concluded that human-mediated wildlife trade, not bat migration, transported these viruses. For SARS-CoV-1, palm civets and raccoon dogs—common in live-animal markets—likely bridged the gap between bats and humans.
“The viruses most closely related to the original SARS coronavirus were found in palm civets and raccoon dogs in southern China, hundreds of miles from the bat populations that were their original source,” said co-senior author Michael Worobey, Ph.D., professor at the University of Arizona. “We’re seeing exactly the same pattern with SARS-CoV-2.”
Study Significance
The study underscores the transnational risk of wildlife trade in driving zoonotic spillovers. Continuous surveillance of sarbecoviruses in bats and high-risk wildlife, particularly in northern Laos and southwestern China, is critical to preventing future outbreaks.
“This international collaboration resolves long-standing questions about the origins of SARS coronaviruses,” said co-senior author Joel Wertheim, Ph.D. “It shows how pathogens can travel far beyond their natural reservoirs through human activities.”