HiMedia is dedicated to maintaining high safety, quality, and performance standards for in-vitro diagnostic products used in healthcare. This commitment significantly enhances the well-being of patients nationally and globally.
Since 2004, HiMedia Laboratories has adopted internationally recognized standards, specifically ISO 13485 Quality Management Systems, which meet the statutory and regulatory requirements for medical and in-vitro diagnostic devices.
HiMedia's certifications from WHO GMP, along with ISO 9001:2015 and ISO 13485:2016 Quality Management Systems, ensure complete traceability of in-vitro diagnostic products throughout their lifecycle - from inception and development to realization and decommissioning.
What are In-Vitro Diagnostic (IVD) Medical Devices?
In-Vitro Diagnostic Medical Devices (IVD products) are instruments and systems designed to diagnose, treat, or prevent medical conditions. They collect and analyze human biological samples, such as urine, feces, blood, saliva, body fluids, tissues, secretions, and swabs.
Samples can be obtained from the nose, throat, veins, or via a fingerstick.
It is essential to understand that IVD products are non-invasive. Nevertheless, they fall under the category of medical devices and are thus subject to the same regulatory controls. They are commonly referred to as IVD Kits, Reagents, or in-vitro diagnostic substances.
Are In-Vitro Diagnostic products regulated in India?
All in-vitro diagnostic kits/reagents are regulated in India under the Medical Devices Rules (2017) and Medical Devices Amendment (2020).
Manufacturing licenses are provided by the Central Drugs Standard Control Organisation (CDSCO) under the Directorate General of Health Services, Ministry of Health & Family Welfare, and the Government of India.
If the manufacturer has fulfilled Indian regulations, do they need to fulfill other rules?
Manufacturers of in-vitro diagnostics must meet the regulatory requirements of the destination country they want to penetrate and supply the products.
Are the regulations the same in India and other countries?
Although their purposes may be similar, they are regulated by their respective National authorities under the provisions of their acts/ regulations.
An intermediate legal entity and the authorized representative acts locally on behalf of a non-native supplier of IVD products.
Global recognition of HiMedia as one of the leading manufacturers & suppliers of IVD products
Products are registered in different countries, fulfilling their medical device regulations. Himedia exports products in more than 150 countries.
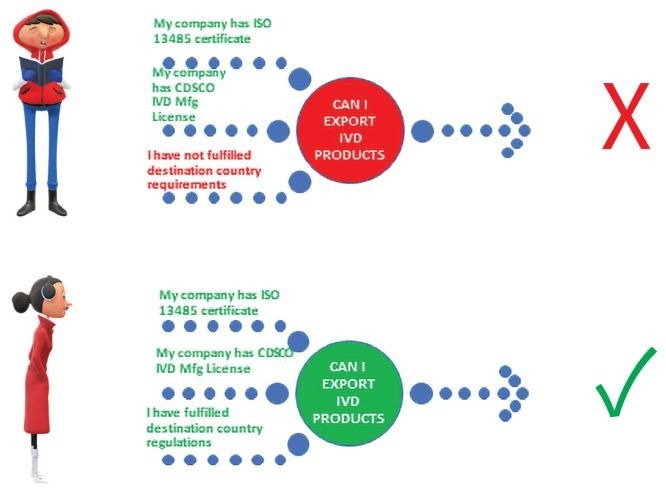
Image Credit: Himedia Laboratories Private Limited
Why are IVD products regulated?
If the results from IVD products are inaccurate, it can adversely affect patient health and present certain risks. To mitigate potential adverse events, IVD products are subject to regulation.
Risk levels associated with IVD products have been universally categorized by the World Health Organization (WHO) as Low-risk, Moderate-risk, and High-risk, based on their potential impact on public health and the individual using the device.
What is risk-based classification of IVD products?
IVDs are categorized based on the health risks they may pose, either to the public or to an individual, due to incorrect results.
Source: Himedia Laboratories Private Limited
| Classification of IVD products |
Risk Level |
| Class A |
Public health and personal risk low |
| Class B |
Public health risk Low; Personal risk moderate to low |
| Class C |
Public health risk moderate - low; Personal risk low |
| Class D |
High Public health and Personal risk |
Registration under Indian MDR
In India, applications are submitted online in the SUGAM portal for the grant of license and registration of medical devices and in vitro diagnostic products by the Central Drugs Standard Control Organization (CDSCO).
IVD are classified as Class A, B, C, or D as per IMDR 2017 Risk- Based Classification Approach. Image Credit: Himedia Laboratories Private Limited
Source: Himedia Laboratories Private Limited
| . |
. |
| CLA (Central Licensing Authority) Conducts audit for Class C & D applications and is authorized to grant licenses of these high-risk group products |
SLA (State Licensing Authority) Conducts audit for Class A & B applications and is authorized to grant licenses of these low & moderate risk group products |
Only SLA (State Licensing Authority) appoints Notified Bodies to carry out this activity. For Class A audit, FDA authorities or Notified Body on behalf of SLA may inspect plant for its approval.
Registration under EU IVDR
For a non-European manufacturer, the European Authorized Representative is a liaison between the non-European manufacturer and the national competent authorities (Ministries of Health) who assist with medical device and IVD registrations, as required.
IVD are classified as Class A Non-sterile & Sterile, B, C, or D as per IVDR 2017/746 Risk-Based Classification Approach. Image Credit: Himedia Laboratories Private Limited
Source: Himedia Laboratories Private Limited
| . |
. |
| IVDs that fall under Nonsterile Class A are self certified and need to involve Authorized representative for registrations in Member State Competent authority |
IVDR in EU has made it mandatory for IVDs Manufacturers to get approval from Notified Body for Class A sterile, B, C or D class. |
There will be no involvement of an Authorized representative for Class A sterile, B, C, or D risk class products. Devices are registered in EU and CE certificate is granted when the product has proven record of clinical safety and performance of the device.
Recent CDSCO update for Class C or D registrations under IMDR 2017 (Indian Medical Device Rule 2017)
Importers and manufacturers who have already filed the application to the Central Licensing Authority on or before 30 September 2023 can continue to import or manufacture the mentioned devices for a maximum of six months starting from the date of this order or until the Central Licensing Authority makes a decision on the applications, whichever comes first.
The most recent update for registrations under IVDR (In-Vitro Diagnostic Regulation 2017/746)
An extension period for IVD products other than Nonsterile Class A has been granted.
- 26 May 2025 for high-risk IVDs Class D
- 26 May 2026 for high-risk IVVDs Class C
- 26 May 2027 for low-risk IVDs Class B & Class A Sterile
Gradual Roll out of EUDAMED, the new electronic database
There is no existing central safety database of in vitro diagnostic products in India accessible to the public. In contrast, the EU has the European Databank on Medical Devices (EUDAMED).
EUDAMED is the IT system developed by the European Commission to implement provisions of Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnosis medical devices.
EUDAMED is a new European system for medical devices that aims to assemble all aspects of registering, monitoring, and managing information about products among different industry actors.
EUDAMED comprises six modules: actor registration, unique device identification (UDI) and device registration, notified bodies and certificates, clinical investigations and performance studies, and vigilance and market surveillance.
Though timelines for product registrations are relaxed, IVD manufacturers and suppliers of IVD products to the EU are required to fulfil new rules.
EU Commission 2010/227 has enforced an electronic database, EUDAMED, wherein it is expected to:
- Provide product information via the completed EUDAMED modules
- Mandatory registration in EUDAMED is expected to begin late 2025
Once up and running, EUDAMED will be able to act as a public registration system, a collaborative system, a notification system, and a dissemination system, and it will be interoperable with other existing and upcoming systems managed by the European Commission, EMA, and Member States.
References
- Central Drugs Standard Control Organization (CDSCO), India. In‑Vitro Diagnostics. Available at: https://cdsco.gov.in/opencms/opencms/en/Medical-Device-Diagnostics/InVitro-Diagnostics
- Central Drugs Standard Control Organization (CDSCO), India (2022). Annexure III IVD FAQs. Available at: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/IVD/FAQs/AnnexureIIIVD.pdf
- European Union (2017). Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices, Official Journal L 117, p. 176–332. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0746
- European Union (1998). Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices, consolidated 7 August 2009. Available at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1998L0079:20090807:en:PDF
- European Commission (2023). EUDAMED roadmap, October 2023. Available at: https://health.ec.europa.eu/system/files/2023-10/md_eudamed_roadmap_en.pdf
- Public Health. (2025). Medical Devices - EUDAMED. (online) Available at: https://health.ec.europa.eu/medical-devices-eudamed/ (Accessed 9 Jun. 2025).
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is amongst the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world renowned for manufacturing high quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated towards products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.