Microcarrier-based platforms have become essential for scalable cell culture across a range of applications—from vaccine production to cell therapy and cultured meat. These systems typically follow a progression through attachment, growth, and detachment phases.
While viable cell density (VCD) monitoring using capacitance sensors is already well-established and aligned with Process Analytical Technology (PAT) guidelines for process optimization and control, real-time monitoring of the detachment phase continues to be a challenge.
Currently, assessing cell detachment often depends on off-line sampling and microscopy—methods that interrupt workflow, reduce precision, and increase the risk of contamination, especially as production scales up. To address this, ACIB Co. explored the use of in-line capacitance-based monitoring to track MA 104 cell detachment from Cytodex 1 microcarriers, leveraging Hamilton’s Incyte sensor.
This real-time, in-line approach provided immediate visibility into detachment dynamics, enabling faster, data-driven decisions and improving overall process consistency. By supporting automated, continuous monitoring, capacitance sensing enhances PAT’s effectiveness in scaling microcarrier-based cultures—not only for vaccines, but also for human mesenchymal stem cell (hMSC) and cultured meat production.
In-situ monitoring of cell detachment from microcarrier by Incyte sensor
The real-time permittivity signal produced by the Incyte sensors was assessed during the detachment of MA 104 cells from Cytodex 1 microcarriers, carried out in a 1-liter DASGIP bioreactor system (Eppendorf, DASGIP® Bioblock parallel system, 700 mL working volume) (Figure 1).
Data acquisition was carried out during the cell detachment process (at a maximum of one hour), using two different generations of Incyte Arc sensors (Incyte Unit DN12) and Incyte Arc Expert (Figure 2) with different final concentrations of Trypsin-EDTA solution as the dissociation reagent.
The Incyte Arc Expert incorporates an integrated preamplifier, which represents a major improvement in design. The capacitance measurements were carried out at a frequency of 1 MHz for all tests, a parameter enhanced for use in mammalian cells.
During the detachment process, off-line samples were systematically gathered to examine cell density and viability utilizing the Trypan Blue assay and predicting the detachment efficiency. Full dissociation of cells from microcarriers was checked via microscopic examination.

Figure 1. Hamilton’s Incyte sensors. Image Credit: Hamilton Bonaduz AG
Result
The results shown in Figure 3 illustrate both the detachment efficiency (Figure 3a) and the corresponding changes in the permittivity signal captured by the Incyte sensor (Figure 3b) during the cell detachment process across varying final Trypsin concentrations.
Following enzyme addition, the permittivity signal dropped sharply within the first five minutes. As supported by the off-line data in Figure 3a, Figure 3b shows that complete cell detachment from the microcarriers (MCs) consistently led to a decline in permittivity below 10 pF/cm. Higher enzyme concentrations (>0.82 mg/mL) produced a more rapid and significant drop in the permittivity signal compared to lower concentrations (0.64 mg/mL).
In contrast, at very low concentrations (0.43 mg/mL), the signal initially declined—primarily due to dilution—but failed to reach the same low values, instead leveling off at a higher plateau.
These real-time permittivity measurements during in-situ detachment highlight the Incyte sensor’s potential for monitoring enzyme activity and predicting detachment outcomes.
A predictive model was developed using permittivity data recorded between minutes 4 and 7 of the detachment phase. This model forecasts the signal at 20 minutes, offering a reliable early indicator of final detachment success. Figure 4 presents the model’s predictive accuracy, with a Root Mean Square Error (RMSE) of 4.6.
To assess signal reproducibility and consistency, additional experiments were carried out using the Incyte Arc sensor at enzyme concentrations of 0.86 g/L and 0.43 g/L.
As shown in Figure 5, the results closely aligned with previous measurements. The main distinction was a steeper signal decline observed with the Arc sensor, suggesting improved responsiveness likely due to enhanced sensitivity in the updated technology.
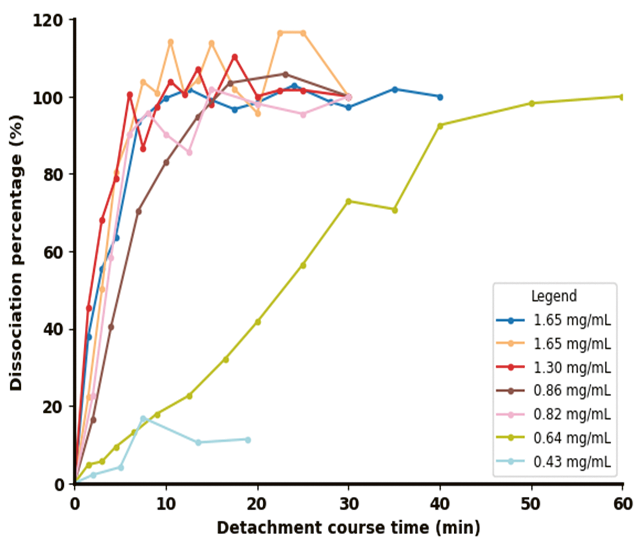
Figure 2a. Cell dissociation monitoring by off-line sampling. Image Credit: Hamilton Bonaduz AG
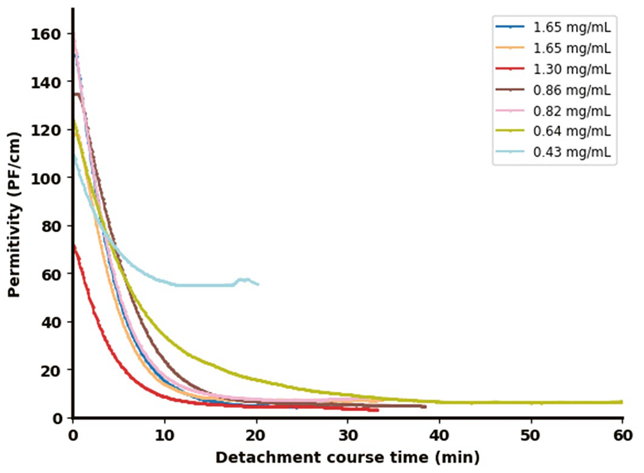
Figure 2b. Impedance probe in bioreactor. Image Credit: Hamilton Bonaduz AG
The experiments demonstrated
Implementing real-time monitoring of cell detachment using a capacitance sensor proved effective in evaluating detachment efficiency and predicting detachment status. This capability enables faster, more informed decision-making for subsequent process steps and enhances overall process control—while also minimizing the need for offline sampling.
The proposed real-time approach is particularly promising for applications involving stem cell detachment from microcarriers, where microcarrier-based expansion has become a well-established and widely adopted method.
Given the sensitivity of stem cells, accurately identifying the optimal timing for enzyme inactivation is critical. Timely intervention can help preserve key surface markers essential for maintaining their differentiation potential.
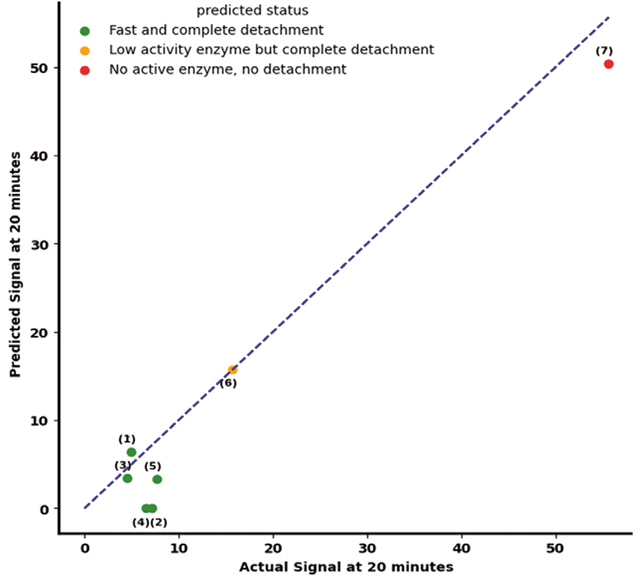
Figure 3. The predictive performance of the developed model using permittivity signals obtained from the Incyte Arc Expert sensor. Image Credit: Hamilton Bonaduz AG
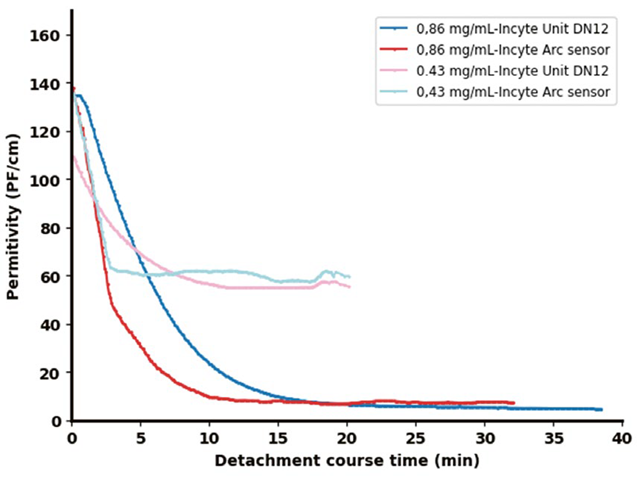
Figure 4. Permittivity signals from two Incyte sensors for two distinct enzyme concentrations: 0.86 g/L and 0.43 g/L. Image Credit: Hamilton Bonaduz AG
About Hamilton Bonaduz AG
The global Hamilton Group, based in Bonaduz and Domat / Ems, Hamilton Bonaduz AG, Hamilton Medical AG, Hamilton Storage GmbH and Hamilton Ems AG, is one of the technology leaders in life science, storage, measurement and medical technology. The success story is based on the invention of the microliter syringe in the late 1940s by American chemical engineer Clark Hamilton. Today Hamilton produces precision instruments for research and industry as well as intelligent ventilators. In addition, the company is strongly represented in the growth markets of genetics and robotics. Currently, the group employs around 3000 people worldwide, more than 1200 of them in Bonaduz and Domat / Ems. The company is wholly owned by the Hamilton family and has been managed by CEO Andreas Wieland since 2001.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.