The Zurich University of Applied Sciences (ZHAW) is one of Switzerland’s leading universities of applied sciences, with core expertise in life sciences and facility management—particularly across the fields of environment, food, and health.

Image Credit: Hamilton Bonaduz AG
The university plays a vital role in addressing social challenges and improving quality of life. Its success is built on five dynamic institutes specializing in chemistry and biotechnology, food and beverage innovation, natural resource sciences, applied simulation, and facility management.
The ZHAW has worked extensively with Hamilton to conduct comprehensive trials and develop the feasibility of its viable cell density (VCD) measurement technology in rocking motion bioreactors.
Hamilton’s VCD measurement technology is widely used in stirred tank bioreactors, but this technology faces challenges in rocking motion bioreactor systems. These reactors often involve the sensing element being only partially covered by the medium, leading to noise in the measurement signal and impacting accuracy and stability.
The ZHAW has worked closely with Hamilton to help mitigate these limitations, contributing to the advancement of bioprocessing technologies in the industry by better optimizing VCD measurement technology for use in rocking motion bioreactors.
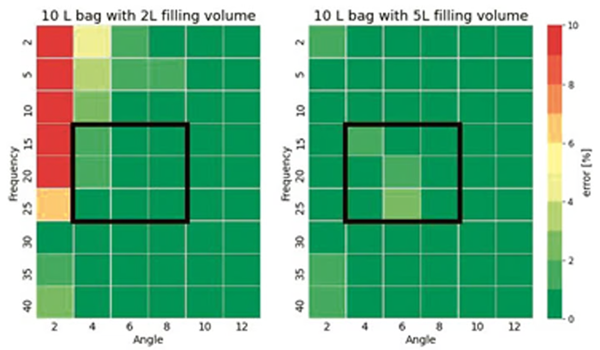
Figure 2. Screening results with 10 L wave bags and two different filling volumes of 2 L and 5 L at different rocking frequency from 2 rpm to 40 rpm and rocking angle from 2° to 12°. A measurement error of up to 4% indicates a permittivity reading within a range from +/-1 pF/cm. Image Credit: Hamilton Bonaduz AG
The bioprocessing industry is moving steadily toward integrated continuous manufacturing, a shift that requires robust process analytical technologies (PAT) such as viable cell density (VCD) measurement. VCD is a key performance indicator because it directly correlates with final product yield by reflecting the number of active, productive cells.
While other parameters—such as product titer, cell viability, product quality attributes, and process efficiency—also shape overall process performance, this article will specifically look at the critical role of VCD.
The ability to reliably measure VCD is impacted by a number of factors, including ensuring that the sensing element is covered with enough media to allow an accurate measurement to be acquired.
Coverage is affected by factors such as bag size, bag filling volume, media properties such as conductivity, and rocking motion speed (which is influenced by the rocker’s rocking angle and the rocks per minute). The patch’s position in the bag also impacts the measurement, though this can be optimized by the bag manufacturer.
Hamilton’s innovative new VCD sensor has been designed to detect favorable measuring conditions, exclude impedance errors, and warn users when process conditions do not allow sufficient measurement accuracy.
Working in collaboration with ZHAW, Hamilton investigated its SU VCD measurement system’s signal stability using Cytiva’s ReadyToProcess Wave 25 Rocker platform and non-sterile custom bags designed to mimic cultivation conditions with sodium chloride solution.
The conductivity measurement best indicates measurement error, so using a conductive solution with zero permittivity was an ideal approach for determining the rocker’s working range.
The Hamilton measurement system demonstrated excellent measurement performance, even at low filling volumes of 20 % of the bag volume. This was also found to be comparable to a competitor’s system in terms of volume and rocking motion speed.
The Hamilton VCD system’s powerful electronics were key to enabling both short measurement cycles and a wider process window.

Figure 3. Wave Bioreactor. Image Credit: Hamilton Bonaduz AG
To validate the theoretical determination of the working range, an IgG-producing ExpiCHO-S cell line (Gibco) was cultivated using the Hamilton VCD system on Cytiva’s ReadyToProcess Wave 25 Rocker platform with sterile custom bags (see Figure 3).
The process began with a 2 L working volume and an initial cell density of 0.3 × 106 cells/mL. Over the 8-day run, two feedings of 1.5 L each were performed. Online VCD readings were continuously compared with offline measurements obtained using a cell culture analyzer (see Figure 4).
Once the VCD dropped below 0.5x106 cells/mL, the process was terminated. This allowed data collection during the stationary and apoptotic phase (death phase) of the cells.
The cultivation process was repeated to highlight the instrument’s precision and to facilitate a comparison of the two processes with the competitor system. It was observed that peak cell densities of 17.5x106 cells/mL and 18.9x106 cells/mL were achieved, respectively (Figure 5).
The peak in the online permittivity during the apoptotic phase appeared due to the misinterpretation of half-dead cells as living cells. This peak can be used as an indication of harvesting time.
The results acquired by working with ZHAW confidently demonstrate that the Hamilton SU VCD measurement system performs well, even at low filling volumes, and that it is able to achieve comparable results to a competitor’s system.
The results also showcase the Hamilton SU VCD measurement system’s capacity to perform with as little as 20 % of the bag volume. The online VCD signal was found to correspond well with the offline measured values during the two cultivations investigated.
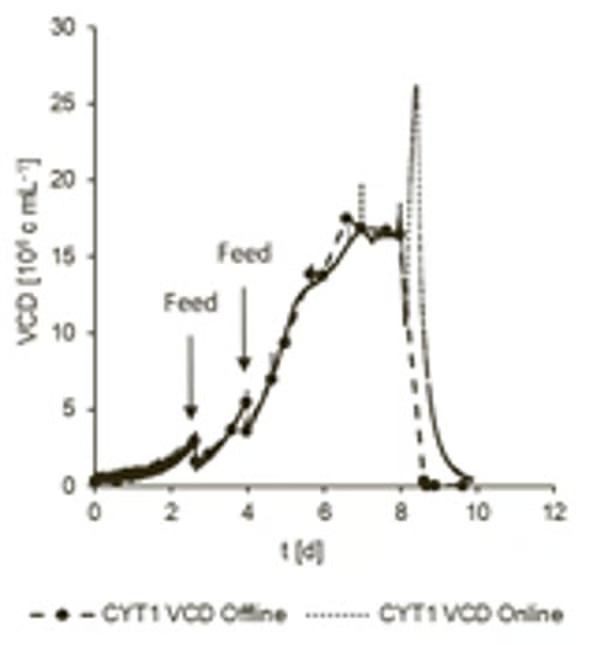
Figure 4. Comparison of online and offline viable cell density with Hamilton system (CYT1). Image Credit: Hamilton Bonaduz AG
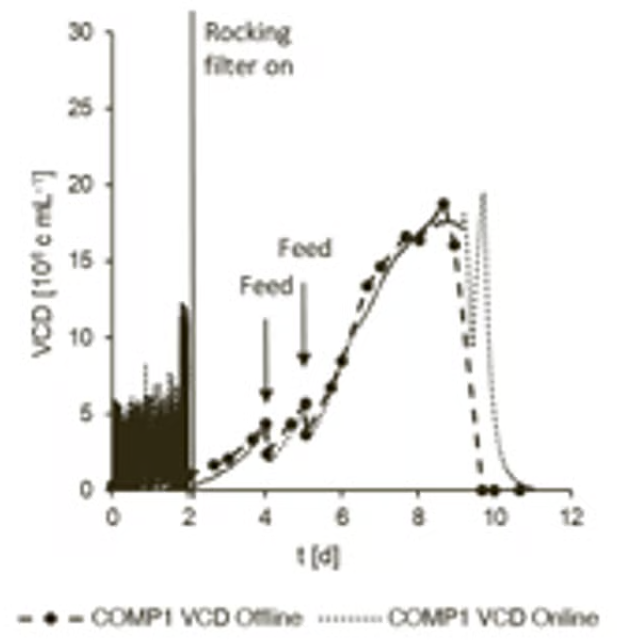
Figure 5. Comparison of online and offline viable cell density with Competitor system (COMP1). Image Credit: Hamilton Bonaduz AG
Hamilton’s Incyte P SU sensor is an essential tool for bioprocessing applications. Its robust and hardwearing construction is coupled with an intuitive design for easy plug and play handling.
Used in combination with the user-friendly ArcAir software, the Incyte P SU sensor enables real-time monitoring of viable cell density during cultivation, leading to enhanced bioprocess controllability and performance.
Acknowledgments
This article is based on materials originally authored by Géraldine Gubser, Yannick Senn, and Iris Poggendorf from the Zurich University of Applied Sciences (ZHAW), School of Life Sciences and Facility Management, Institute of Chemistry and Biotechnology.
About Hamilton Bonaduz AG
The global Hamilton Group, based in Bonaduz and Domat / Ems, Hamilton Bonaduz AG, Hamilton Medical AG, Hamilton Storage GmbH and Hamilton Ems AG, is one of the technology leaders in life science, storage, measurement and medical technology. The success story is based on the invention of the microliter syringe in the late 1940s by American chemical engineer Clark Hamilton. Today Hamilton produces precision instruments for research and industry as well as intelligent ventilators. In addition, the company is strongly represented in the growth markets of genetics and robotics. Currently, the group employs around 3000 people worldwide, more than 1200 of them in Bonaduz and Domat / Ems. The company is wholly owned by the Hamilton family and has been managed by CEO Andreas Wieland since 2001.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.