Oncolytic viruses, including the measles virus (MeV), exhibit a preferential ability to kill cancer cells, making them promising candidates for novel tumor therapies.
As part of their infection cycle, an oncolytic virus enters a cancer cell and rewires the cell’s synthetic machinery to produce viral components. Viral particles are synthesized until the cell eventually bursts, leading to the death of the host cell and the release of new infectious particles, which then reinitiate the infection cycle.
Once released into the culture medium, however, viral particles gradually lose their infectivity over time. This creates a critical challenge in the biomanufacturing of infectious viral particles for therapeutic use: accurately determining the optimal time of harvest (TOH). Harvesting too early limits yield, while harvesting too late risks a decline in viral potency.
In the context of biotechnological processes focused on producing oncolytic viruses for therapeutic applications, achieving the right balance is essential. Manufacturers must maintain viable host cells to support ongoing virus production while also harvesting at the peak of viral infectivity. This balance is fundamental for ensuring both process efficiency and the quality of the therapeutic product.
Aim: Determine the best time of harvest (TOH) to maximize the yield of infectious virus particles.
Traditionally, viral infectivity is assessed using methods such as plaque assays or TCID50 (median tissue culture infectious dose) assays.
While well established, these techniques are time-consuming to set up, require incubation periods of several days, and carry a risk of contamination due to the repetitive, manual nature of the procedures. Most importantly, they are not suited for real-time, in-process analysis—making them inadequate for determining the optimal TOH during active virus production.
Challenge: Traditional offline methods are time-consuming and subjective.
To address these limitations, the working group Cell Culture of the Institute of Bioprocess Engineering and Pharmaceutical Technology (IBPT) at the University of Applied Sciences Mittelhessen (THM) applies process analytical technology (PAT) for the development of novel processes in mammalian cell culture.
One PAT tool currently under investigation is dielectric spectroscopy (DS).
DS is a non-invasive analytical technique that measures a cell’s permittivity—its ability to become polarized when exposed to an electric field. This parameter provides valuable insights into cell viability, growth, and changes in cellular composition or morphology, all of which are critical factors in virus production.
As permittivity relies on intact cell membranes for polarization, DS selectively measures viable cells, distinguishing them from non-living components such as dead cells, debris, or scaffolds that may be present in culture.
Overall, DS represents a powerful tool for optimizing cell culture conditions and improving virus production yields.
Solution: Identify an in-line PAT tool capable of delivering objective, real-time data with the flexibility to integrate data analysis software and automated control loops.
In this study, measles virus (MeV) was produced in Vero cells cultivated on Cytodex 1 microcarriers (3 g/L) within a 1 L stirred tank reactor (STR) equipped with a siliconized glass vessel and a 0.5 L working volume.
The culture conditions were carefully controlled: the temperature was maintained at 32 °C, the pH was regulated to 7.4 ± 0.1 using 1 M NaOH or CO2 gassing, and the stirrer speed was set at 70 rpm.
Dissolved oxygen levels were kept between 50–70 % through discontinuous aeration (0.01 vvm), as earlier studies had shown that continuous aeration negatively impacts virus titer.1
The concentration of viable cells was monitored in-line using dielectric spectroscopy (DS) with Hamilton’s Incyte sensor and ArcView system, measuring permittivity at 1 MHz with an F-scan range of 0.3–10 MHz.
For comparison, total cell concentration was determined offline by dissolving cells bound to microcarriers with crystal violet solution (1 g/L in 0.1 % [w/v] citric acid) and counting cell nuclei with a Neubauer counting chamber. Viral infectivity was subsequently assessed by TCID50 assay from daily frozen reactor samples.
This approach offers a number of advantages.
- Dielectric spectroscopy (DS) is ideally suited to measuring viable cell density in uninfected Vero cells in real time.
- The maximum virus titer in the supernatant is directly correlated with the global permittivity maximum, occurring with a time offset.
- It is possible to use DS to determine the timeframe from viral infection to cell lysis, enabling predictions of optimal TOH.

Figure 2. Impedance probe in bioreactor. Image Credit: Hamilton Bonaduz AG
Results
In uninfected Vero cells, the permittivity signal correlated closely with total cell concentration determined offline, beginning with the cell adhesion phase (approximately 4–7 hours post-inoculum) and continuing through the exponential growth phase (up to ~50 hours post-inoculum).
In contrast, no such correlation was observed in Vero cells infected with MeV. At a high multiplicity of infection (MOI) of 30 TCID50 per cell, the permittivity signal increased during the first 0–48 hours post-infection, while the total cell concentration measured offline remained nearly constant (Figure 3).
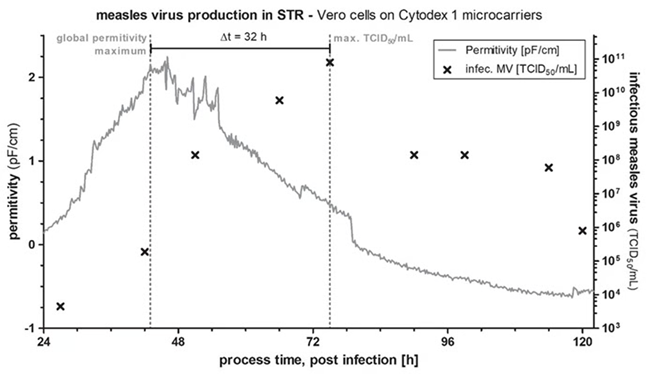
Figure 3. Relationship between global permittivity measurement using Incyte and infectious virus titer. Image Credit: Hamilton Bonaduz AG
After this period, the permittivity signal plateaued briefly before declining steadily (Figure 3).
This time course aligns with the microscopic observations of Grein et al.,2 who reported characteristic morphological changes in MeV-infected Vero cells under high MOI.
Their findings showed the formation of syncytia within the first 48 hours post-infection, persisting for up to 24 hours, followed by detachment of infected cells from the microcarrier surface and eventual cell lysis.
Conclusion
Although DS cannot be applied to determine cell concentration in MeV-infected Vero cells, it can capture morphological changes, enabling the identification of the time frame for syncytia formation and the onset of cell lysis. This capability allows DS to be used as a predictive tool for determining the optimal TOH.
An evaluation of permittivity data from 16 independent MeV productions in the STR, combined with corresponding virus titers from daily reactor samples (measured by TCID50 assay), showed that maximum virus titers in the supernatant occurred 39.6 ± 7 hours after the global permittivity maximum.2 Additional experiments with shorter sampling intervals may further improve the precision of TOH prediction.
Acknowledgments
Produced from materials originally authored by Hamilton Bonaduz AG.
References and further reading
- Grein, T.A., et al. (2019). Aeration and Shear Stress Are Critical Process Parameters for the Production of Oncolytic Measles Virus. Frontiers in Bioengineering and Biotechnology, 7. https://doi.org/10.3389/fbioe.2019.00078.
- Grein, T.A., et al. (2018). High titer oncolytic measles virus production process by integration of dielectric spectroscopy as online monitoring system. Biotechnology and Bioengineering, 115(5), pp.1186–1194. https://doi.org/10.1002/bit.26538.
About Hamilton Bonaduz AG
The global Hamilton Group, based in Bonaduz and Domat / Ems, Hamilton Bonaduz AG, Hamilton Medical AG, Hamilton Storage GmbH and Hamilton Ems AG, is one of the technology leaders in life science, storage, measurement and medical technology. The success story is based on the invention of the microliter syringe in the late 1940s by American chemical engineer Clark Hamilton. Today Hamilton produces precision instruments for research and industry as well as intelligent ventilators. In addition, the company is strongly represented in the growth markets of genetics and robotics. Currently, the group employs around 3000 people worldwide, more than 1200 of them in Bonaduz and Domat / Ems. The company is wholly owned by the Hamilton family and has been managed by CEO Andreas Wieland since 2001.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.