Microcarrier-based platforms have transformed scalable cell culture for a wide range of applications, from cell therapy and vaccine production to cultured meat.
Viable cell density (VCD) monitoring using capacitance sensors aligns with Process Analytical Technology (PAT) guidelines, making it a strong choice for optimizing and controlling cell cultures throughout the attachment, growth, and detachment phases.
Real-time monitoring of cell detachment remains challenging, however, despite being critical for timely bioreactor transfer and scale-up. This is because conventional monitoring methods are reliant on off-line sampling and microscopy, disrupting workflows, limiting precision, and increasing contamination risk, particularly at larger scales.
Working with the Austrian Center of Industrial Biotechnology (ACIB), Hamilton leveraged in-line capacitance-based monitoring to track MA 104 cell detachment from Cytodex 1 microcarriers using Hamilton’s Incyte sensor. This integration provided real-time insights into the detachment process, enabling immediate decision-making and improved process consistency.
By supporting automated, continuous data collection, capacitance monitoring strengthens the role of PAT in scaling microcarrier cultures, with applications extending beyond vaccines to include hMSC and cultured meat production.
In-situ monitoring of cell detachment from microcarrier via Incyte sensor
An investigation was done into the real-time permittivity signal generated by the Incyte sensors during the detachment of MA 104 cells from Cytodex 1 MCs.
A 1-liter DASGIP bioreactor system (Eppendorf, DASGIP® Bioblock parallel system, 700 mL working volume) (Figure 1) was used in this study, with data acquired throughout the cell detachment process for a maximum of 1 hour.
This was done using two different generations of Incyte Arc sensors (Figure 2)–the Incyte Unit DN12 and the Incyte Arc Expert–with different final concentrations of trypsin-EDTA solution employed as the dissociation reagent. The Incyte Arc Expert features significant design enhancement in the form of an integrated preamplifier.
Capacitance measurements were acquired at a frequency of 1 MHz for all experiments. This parameter had been optimized for mammalian cell applications.
Off-line samples were systematically collected during the detachment process to evaluate cell density and viability. This was done using the Trypan Blue assay and via detachment efficiency calculations. Microscopic examination was used to verify the complete dissociation of cells from microcarriers.

Figure 1. Bioreactor set up with Hamilton’s Incyte sensor. Image Credit: Hamilton Bonaduz AG
Results
The results shown in Figure 3 highlight detachment efficiency (Figure 3a) and the evolution of the permittivity signal from the Incyte sensor (Figure 3b) during the cell detachment process for different final concentrations of trypsin.
The permittivity signal showed a pronounced decline in the first 5 minutes after adding the enzyme solution. Using the off-line measurements shown in Figure 3a and the permittivity signal shown in Figure 3b, it is evident that complete cell detachment from the MCs prompted a decrease in the permittivity signal to levels below 10 pF/cm.
It was also observed that higher enzyme concentrations (> 0.82 mg/mL) led to a more rapid and pronounced decrease in permittivity signal versus lower concentrations (0.64 mg/mL).
It was also noted that, while the signal initially declined due to dilution of the solution, at very low enzyme concentrations (0.43 mg/mL), it failed to achieve the same minimal permittivity values, instead stabilizing at a higher plateau.
The results from the on-line permittivity signal measurement during in situ cell detachment highlight the Incyte sensor’s potential as a tool for monitoring enzyme activity and predicting the chances of complete cell detachment.
It was possible to establish a predictive model using permittivity signals recorded between minutes 4 and 7 of the detachment process. This model could be used to forecast the permittivity signal at 20 minutes to help predict the final cell detachment outcome.
Figure 4 shows the developed models’ predictive accuracy (Root Mean Square Error (RMSE) = 4.6) and performance.
Experiments were done using the Incyte Arc sensor at enzyme concentrations of 0.86 g/L and 0.43 g/L in order to validate the consistency and reproducibility of the signal obtained via the Incyte sensors.
Figure 5 compares these results, confirming similar outcomes, with the key difference being a steeper decline noted with the Incyte Arc sensor. This more rapid response highlights improved sensor technology, most likely due to improved sensitivity.
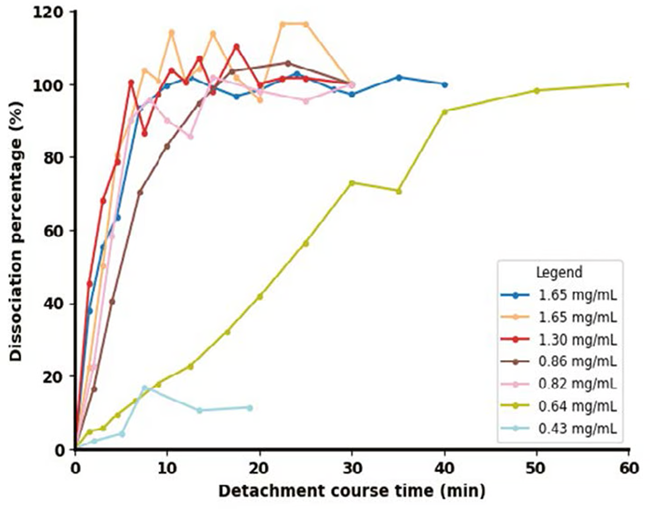
Figure 3a. Cell dissociation monitoring by off-line sampling. Image Credit: Hamilton Bonaduz AG
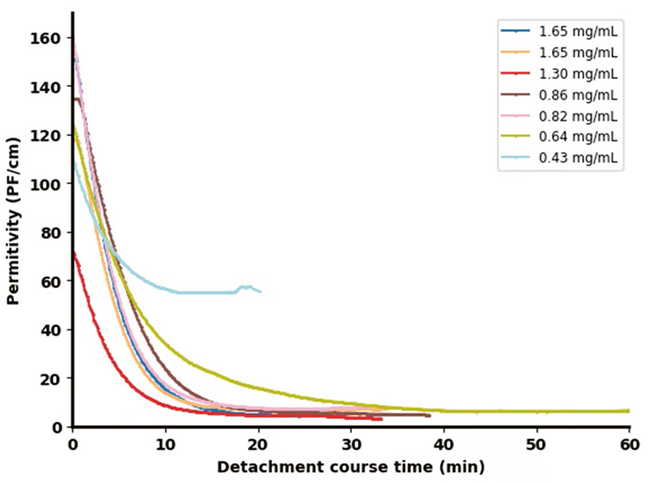
Figure 3b. Impedance probe in bioreactor. Image Credit: Hamilton Bonaduz AG
All in all, the use of real-time cell detachment monitoring with a capacitance sensor proved highly effective in evaluating detachment efficiency and predicting detachment status. This capability enables timely decision-making for subsequent process steps, enhances overall control, and reduces reliance on offline sampling.
The method also showed strong potential for applications involving stem cell detachment from microcarriers, where microcarrier-based cell propagation has become an established and widely accepted practice.
Given the sensitivity of stem cells, accurately determining the optimal timing for enzyme solution inactivation is critical. Real-time monitoring helps prevent damage to essential surface markers, which are vital for maintaining the cells’ differentiation capacity.
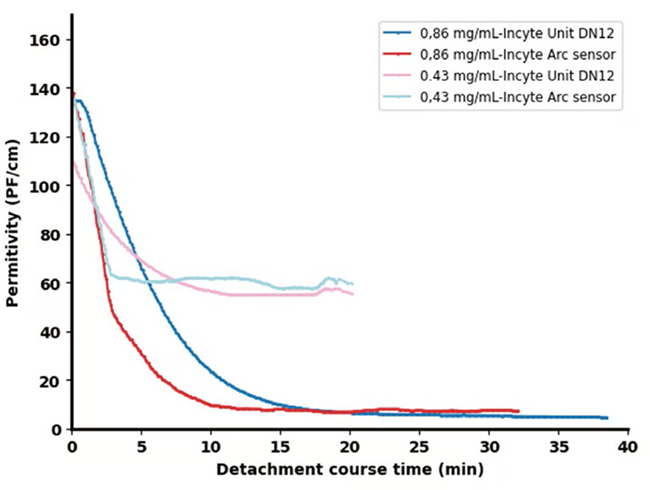
Figure 4. The predictive performance of the developed model using permittivity signals obtained from the Incyte Arc Expert sensor. Image Credit: Hamilton Bonaduz AG
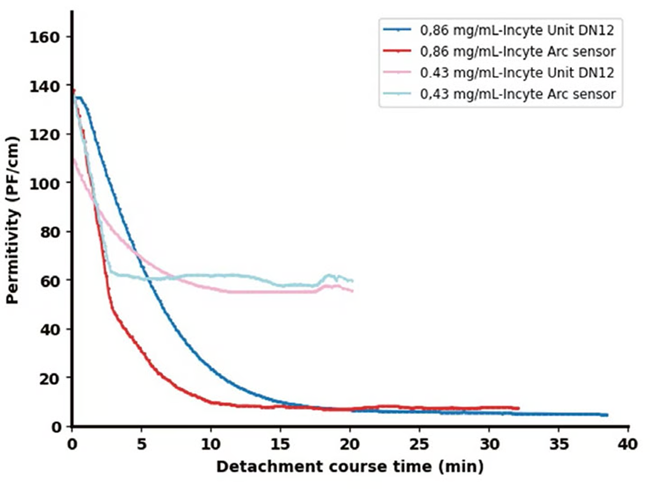
Figure 5. Permittivity signals from two Incyte sensors for two distinct enzyme concentrations: 0.86 g/L and 0.43 g/L. Image Credit: Hamilton Bonaduz AG
Acknowledgments
Produced from materials originally authored by Atefeh Ebrahimian and Harald Kühnel, Austrian Center of Industrial Biotechnology (ACIB), FH Campus Wien.
About Hamilton Bonaduz AG
The global Hamilton Group, based in Bonaduz and Domat / Ems, Hamilton Bonaduz AG, Hamilton Medical AG, Hamilton Storage GmbH and Hamilton Ems AG, is one of the technology leaders in life science, storage, measurement and medical technology. The success story is based on the invention of the microliter syringe in the late 1940s by American chemical engineer Clark Hamilton. Today Hamilton produces precision instruments for research and industry as well as intelligent ventilators. In addition, the company is strongly represented in the growth markets of genetics and robotics. Currently, the group employs around 3000 people worldwide, more than 1200 of them in Bonaduz and Domat / Ems. The company is wholly owned by the Hamilton family and has been managed by CEO Andreas Wieland since 2001.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.