2. What should I know before I use ADVATE?
Do not use if you have ever had sudden, severe or potentially life-threatening allergic
reaction to any of the ingredients listed at the end of the CMI or to mouse or hamster
proteins. Talk to your doctor if you have or have had any other medical conditions,
if you have had or at risk of any heart problems, if you take any other medicines,
or if you are pregnant or plan to become pregnant or if you are breastfeeding or plan
to breastfeed. For more information, see Section
2. What should I know before I use ADVATE? in the full CMI.
3. What if I am taking other medicines?
Tell your doctor or Haemophilia Treatment Centre if you are taking or using any other
medicines including any that you get without a prescription from your pharmacy, supermarket,
or health food shop. For more information, see Section
3. What if I am taking other medicines? in the full CMI.
4. How will I be given ADVATE?
ADVATE injection will be prepared and given to you by a qualified healthcare professional
who is experienced in the care of patients with haemophilia. Some individuals (and/or
their caregivers) may be trained to use ADVATE at home.
Your doctor will decide on your dose of ADVATE depending on your condition and body
weight.
The frequency of infusions you receive, and how long you will use ADVATE for, will
depend on how well ADVATE works for you. Your doctor may change the dose you use during
your treatment.
ADVATE is slowly injected directly into your veins.
Before injection, the ADVATE powder must be mixed and dissolved using the diluent
supplied.
5. What should I know while using ADVATE?
|
Things you should do
|
Tell your doctor or Haemophilia Treatment Centre immediately if you notice
- any sudden signs and symptoms of a severe allergic reaction.
- your bleeding is not controlled or worsens after using ADVATE.
Tell any doctors, dentists, or pharmacists you visit that you are using ADVATE or
if you are about to have any blood tests.
Keep all your appointments with your doctor and any blood tests.
|
|
Things you should not do
|
Do not give your medicine to anyone else, even if they appear to have the same condition
as you.
Do not stop using your medicine or change the dosage without checking with your doctor.
|
|
Looking after your medicine
|
Keep ADVATE in the pack until it is time to use it so that it is protected from light.
Keep ADVATE in the refrigerator at 2°C to 8°C. Do not freeze.
|
6. Are there any side effects?
Very common side effects include factor VIII inhibitors against the medicine (in patients
who have not been previously treated with factor VIII medicines). Common side effects
include headache and fever. Serious side effects include sudden signs and symptoms
of a severe allergic reaction which may include rash, hives, itching, swelling of
your face, lips and tongue, shortness of breath, wheezing, difficulty in breathing,
tightness or discomfort in the chest. For more information, including what to do if
you have any side effects, see Section
6. Are there any side effects? in the full CMI.
Active ingredient(s):
octocog alfa
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using ADVATE.
You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using ADVATE.
Where to find information in this leaflet:
1. Why am I using ADVATE?
ADVATE contains the active ingredient octocog alfa.
ADVATE is used for the management of congenital haemophilia A (an inherited bleeding
disorder caused by a lack of factor VIII in the blood).
ADVATE can be used in patients of all age groups with haemophilia A to:
control and prevent bleeding episodes,
routinely prevent and reduce the frequency of bleeding episodes,
prevent or reduce bleeding before, during and after surgery.
The blood clotting factor VIII is essential for the blood to form clots and stop bleedings.
Patients with congenital haemophilia A are born with a low level of factor VIII (either
missing or not working properly) in the blood circulation.
ADVATE is a man-made copy of the blood clotting factor VIII in human blood. It is
produced by recombinant DNA technology. ADVATE works similarly to the human blood
clotting factor VIII and by replenishing the factor VIII levels in haemophilia A patients,
blood can form clots at the site of bleeding.
This medicine helps to control your condition but does not cure it.
Ask your doctor if you have any questions about why this medicine has been prescribed
for you.
Your doctor may have prescribed it for another reason.
2. What should I know before I use ADVATE?
Warnings
Do not use ADVATE if you:
have ever had sudden, severe or potentially life-threatening allergic reaction to
octocog alfa or any of the ingredients listed at the end of this leaflet,
have ever had sudden, severe or potentially life-threatening allergic reaction to
mouse or hamster proteins or if you have a known allergy to medicines of mouse or
hamster origin.
Always check the ingredients to make sure you can use this medicine. If you are unsure
about this, ask your doctor.
Check with your doctor if you:
have been previously treated with factor VIII products.
have previously developed inhibitors to factor VIII products.
Inhibitors to factor VIII are blocking antibodies that can work against ADVATE and
reduce the ability of ADVATE to prevent or control bleeding.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. There is
no information on the use of ADVATE during pregnancy. Your doctor will discuss the
risks and benefits of using ADVATE if you are pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known
if ADVATE passes into your milk and if it can harm your baby. Your doctor will discuss
the risks and benefits of using ADVATE if you are breast-feeding.
3. What if I am taking other medicines?
Tell your doctor or Haemophilia Treatment Centre if you are taking any other medicines,
including any medicines, vitamins or supplements that you buy without a prescription
from your pharmacy, supermarket or health food shop.
There are no known interactions of ADVATE with other medicines.
Your doctor or Haemophilia Treatment Centre have more information on medicines to
be careful with or avoid if you have concerns.
4. How do I use ADVATE?
How much to use
Follow all directions given to you by your doctor carefully. They may differ from
the information contained in this leaflet.
Your doctor will decide how much ADVATE you use.
Your dose will depend on:
- your body weight,
- the amount of factor VIII your body is able to make,
- how much, how often and where you are bleeding,
- whether your body have built up antibodies to ADVATE,
- whether you are using ADVATE to prevent or treat bleeding.
Your doctor will tell you how often or at what intervals you will receive the injection.
The frequency of injections you receive will depend on how well ADVATE works for you.
How to use ADVATE
Treatment with ADVATE will be started in a hospital or Haemophilia Treatment Centre
and supervised by your doctor or qualified healthcare professional who is experienced
in the care of patients with haemophilia.
After starting ADVATE treatment, some individuals (and/or their caregivers) may be
trained to use ADVATE at home.
ADVATE is injected slowly directly into your vein.
Do not attempt to inject ADVATE at home unless you (and/or your caregiver) have received
proper training by your doctor or Haemophilia Treatment Centre on how to use the product.
Preparing ADVATE
ADVATE is provided as a powder in a vial, and a diluent vial containing water for
injections is also supplied in the pack. Before use, the ADVATE powder must be mixed
and dissolved using the diluent provided to form a clear solution.
Follow carefully the step-by-step instructions at the end of this leaflet or in the
pack insert on how to prepare and inject ADVATE.
Do not mix ADVATE with any other medicines or solvent other than the water for injections
diluent supplied with the pack.
Use only the reconstitution device provided with each pack to prepare the solution
for injection.
After mixing the powder and the diluent, use the solution immediately. If the solution
is not used straight way, you can keep the solution for a maximum of 3 hours when
stored at room temperature.
Do not refrigerate the solution after it is prepared.
ADVATE is for single use in one patient only. Dispose of all unused solution, empty
vials, and used needles and syringes into a sharps bin.
If you are unsure about how to prepare the medicine for use, contact your doctor or
Haemophilia Treatment Centre.
Inspecting ADVATE
Always inspect ADVATE before use and after it has been mixed.
After mixing, the solution should be clear to colourless, and free from foreign particles.
Do not inject the solution if it is discoloured, or cloudy, or contains particles.
How long to use ADVATE
Continue using ADVATE for as long as your doctor tells you.
Usually, replacement therapy with ADVATE is a life-long treatment. The symptoms of
your condition may worsen if you stop treatment.
If you forget to use ADVATE
Do not inject a double dose to make up for the forgotten dose.
If you inject a double dose, this may increase the chance of you getting an unwanted
side effect.
If you are not sure what to do, ask your doctor or Haemophilia Treatment Centre for
advice and continue your next injection as directed.
If you use too much ADVATE
If you think that you have used too much ADVATE, you may need urgent medical attention.
You should immediately:
contact your doctor or Haemophilia Treatment Centre, or
go to the Emergency Department at your nearest hospital, or
phone the Poisons Information Centre by calling 13 11 26 (if you are in Australia), or by calling 0800 764 766 (if you are in New Zealand).
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using ADVATE?
Things you should do
Each time you use ADVATE, keep a record of the name and batch number of the medicine.
Tell your other doctors, dentists and pharmacists you are using this medicine.
If you are about to have any blood tests, tell your doctor that you are using ADVATE.
Keep all your doctor's appointments so that your progress can be checked. Your doctor
will do some blood tests before you start your treatment and from time to time during
your treatment to make sure you have adequate factor VIII levels.
Follow all instructions from your doctor. Your doctor may change the dose you use
using your treatment.
Tell your doctor and/or Haemophilia Treatment Centre straight away if you notice:
any sudden signs and symptoms of a severe allergic response.
your bleeding is not controlled or worsens after using ADVATE.
Things you should not do:
Do not give your medicine to anyone else, even if they appear to have the same condition
as you.
Do not use ADVATE to treat any other complaints unless your doctor tells you to.
Do not stop using ADVATE unless advised by your doctor or healthcare professional
or unless you have an allergic reaction.
Do not change the dosage without checking with your doctor.
Do not use ADVATE after the expiry date which is printed on the label after the word
'EXP'. The expiry date refers to the last day of the month.
Do not store the pack at room temperature for more than 6 months.
Do not use the solution after it is prepared for more than 3 hours.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how ADVATE
affects you.
ADVATE is not expected to have an influence on your ability to drive and use machines.
Looking after your medicine
Keep ADVATE in the pack until it is time to use it. This will protect the medicine
from light.
Keep ADVATE in the refrigerator at 2°C to 8°C in a refrigerator. Do not freeze.
If necessary, you can keep ADVATE out of the refrigerator for a single 6-month period
when stored in the original packaging in a cool dry place at room temperature (below
25°C). Record on the carton the date ADVATE is removed from refrigeration.
Do not return the medicine to the refrigerator after it has been stored at room temperature.
Follow the instructions in the carton on how to take care of your medicine properly.
Keep ADVATE out of reach of children.
Getting rid of any unwanted medicine
Medicines should not be disposed of via wastewater or household waste.
If your doctor tells you to stop using this medicine, or if the medicine is out of
date, or if the medicine has not been stored properly, ask your doctor or Haemophilia
Treatment Centre what to do with any unwanted medicine that is left over.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
In patients receiving ADVATE, it is possible that the body may develop factor VIII
inhibitors which work against the medicine. This risk is very common in patients who
have not been previously treated with factor VIII medicines. In patients who have
received previous treatment with factor VIII medicines (for more than 150 days), this
risk is uncommon. When factor VIII inhibitors build up, ADVATE may stop working properly.
Signs or symptoms may include easily bruising or persistent bleeding, swelling and
pain or tightness in joints. If you feel that your condition is not being controlled
with ADVATE, tell your doctor or Haemophilia Treatment Centre immediately.
Tell your doctor, Haemophilia Treatment Centre, or pharmacist if you notice anything
else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor, Haemophilia Treatment Centre, or pharmacist
before you decide to stop using any of your medicines.
7. Product details
What ADVATE contains
This medicine is only available with a doctor's prescription.
Powder in a vial
|
Active ingredient
(main ingredient)
|
octocog alfa pegol
|
|
Other ingredients
(inactive ingredients)
|
trehalose dihydrate
histidine
mannitol
polysorbate 80
sodium chloride
calcium chloride dihydrate
glutathione
trometamol
|
Diluent in a vial
|
Other ingredients
(inactive ingredients)
|
water for injections
|
Do not take this medicine if you are allergic to any of these ingredients.
What ADVATE looks like
ADVATE is supplied as a white to off-white powder in a single-dose glass vial.
Each pack of ADVATE contains:
1 drug powder vial of ADVATE
1 diluent vial of water for injections (5 mL or 2 mL)
1 reconstitution device (BAXJECT II).
ADVATE is available in 7 strengths:
ADVATE 250 IU (5 mL or 2 mL) - AUST R 100384
ADVATE 500 IU (5 mL or 2 mL) - AUST R 100385
ADVATE 1000 IU (5 mL or 2 mL) - AUST R 100386
ADVATE 1500 IU (5 mL or 2 mL) - AUST R 100387
ADVATE 2000 IU (5 mL) - AUST R 136204
ADVATE 3000 IU (5 mL) - AUST R 150366
ADVATE 4000 IU (5 mL) - AUST R 214709
Not all presentations may be marketed.
Who distributes ADVATE
ADVATE is supplied in Australia by:
Takeda Pharmaceuticals Australia Pty Ltd
Level 39, 225 George Street
Sydney NSW 2000
Australia
Telephone: 1800 012 612
www.takeda.com/en-au
ADVATE is supplied in New Zealand by:
Takeda New Zealand Pty Limited
Level 10, 21 Queen Street
Auckland 1010
New Zealand
Telephone: 0508 169 077
www.takeda.com/en-au
This leaflet was prepared in May 2024.
8. Instructions for use
This instructions for use is intended only for healthcare professionals and for those
patients and/or caregivers who have been trained by their doctor or healthcare professional
on the proper way to self-inject the medicine.
Do not attempt to inject ADVATE by yourself unless you have received proper training
by your doctor or Haemophilia Treatment Centre on how to use the product.
Contact your doctor or Haemophilia Treatment Centre if you have any questions or if
you experience any problems following this instruction guide.
IMPORTANT
Use only the water for injections (diluent) and the reconstitution device provided
in the pack to prepare the solution for injection.
If more than one vial of ADVATE is needed for the dose, mix each vial of ADVATE using
a separate BAXJECT II device supplied in each pack.
After preparing ADVATE, use the solution as soon as possible, within 3 hours after
mixing.
Do not refrigerate the solution after it is prepared.
Always inspect ADVATE before use and after it has been mixed. After mixing, the solution
should be clear to colourless.
Do not use the solution if it is discoloured, or cloudy, or contains particles.
Preparing ADVATE using aseptic technique
In a quiet place, prepare a clean surface and gather all the materials you will need
for the injection.
Remove ADVATE from the refrigerator and check the expiry date on the package.
Wash your hands and put on clean exam gloves. If you are self-injecting at home the
use of gloves is optional.
Using the BAXJECT II device
1. Allow the ADVATE powder and diluent vials to reach room temperature before use.
2. Remove plastic caps from the ADVATE powder and diluent vials.
3. Cleanse rubber stoppers with an alcohol wipe and allow to dry before use.
4. Open the BAXJECT II device package by peeling away the lid, without touching the inside
(Figure A). Do not remove the device from the package.
Figure A
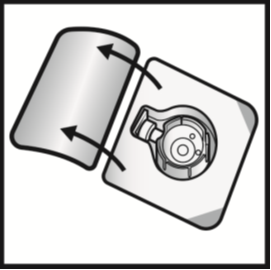
5. Turn the package over. Press straight down to fully insert the clear plastic spike
through the diluent vial stopper (Figure B).
Figure B
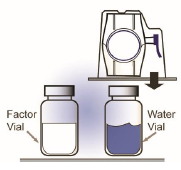
6. Grip the BAXJECT II package at its edge and pull the package off the device (Figure
C). Do not remove the blue cap from the BAXJECT II device. Do not touch the exposed
white plastic spike.
Figure C
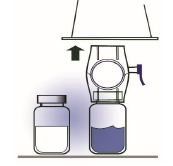
7. Turn the system over so that the diluent vial is on top. Quickly insert the white
plastic spike fully into the ADVATE vial stopper by pushing straight down (Figure
D). The vacuum will draw the diluent into the ADVATE vial.
Figure D
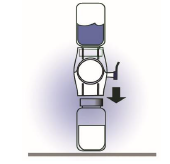
8. Swirl gently until the powder is completely dissolved. DO NOT SHAKE.
Administration
9. Remove the blue cap from the BAXJECT II device. Connect the syringe to the BAXJECT
II (Figure E). DO NOT INJECT AIR into the BAXJECT II device.
Figure E
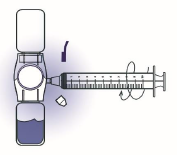
10. Turn the system upside down (ADVATE vial now on top). Draw the reconstituted solution
into the syringe by pulling the plunger back slowly (Figure F).
Figure F
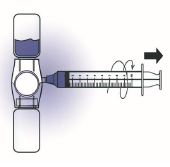
11. Disconnect the syringe, attach a suitable needle and inject slowly into the vein over
a period of up to 5 minutes (maximum infusion rate of 10 mL per minute).
12. If more than one vial of ADVATE is to be used, the contents of multiple vials may
be drawn into the same syringe.
ADVATE® and BAXJECT® are registered trademarks of Baxalta Incorporated.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.