Introduction
BEVs: Structure and function
BEVs in PCOS
BEVs in endometriosis
Clinical potential of BEVs
Research frontiers and limitations
Conclusions
References
Further reading
Bacterial extracellular vesicles (BEVs) play a crucial role in mediating gut-reproductive communication and immune regulation. Understanding their influence on PCOS and endometriosis reveals new possibilities for non-invasive diagnostics and targeted therapies.
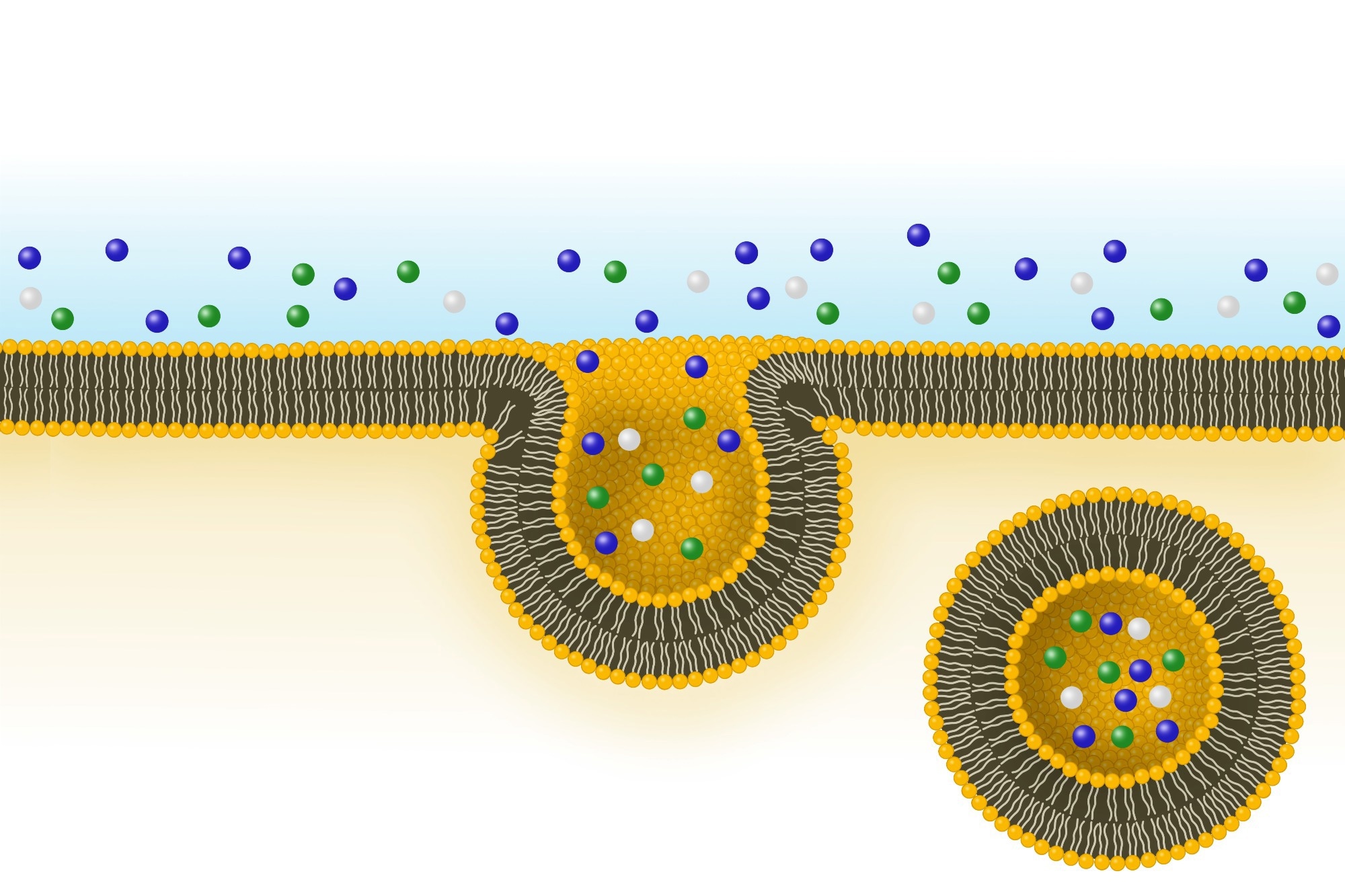 Image Credit: J. Marini / Shutterstock.com
Image Credit: J. Marini / Shutterstock.com
Introduction
Bacterial extracellular vesicles (BEVs) are membrane-bound particles between 20 and 400 nanometers (nm) in size that are secreted by bacteria. BEVs carry bioactive agents that mediate cell-to-cell communication, thereby influencing bacterial survival, virulence, and host signaling pathways. BEVs are released by both Gram-negative and Gram-positive bacteria and have been detected in multiple human biofluids, supporting a role in systemic host–microbe communication.1,3,11,12
BEVs have emerged as key mediators of the ‘gut-ovary axis,’ which connects microorganisms within the gastrointestinal tract to reproductive health. Growing evidence suggests that BEV-driven microbial imbalance contributes to conditions such as polycystic ovary syndrome (PCOS) and endometriosis.2
PCOS and endometriosis, which affect millions of women worldwide, increase the risk of infertility, metabolic complications, and reduced quality of life. By elucidating the physiological roles of BEVs, researchers can better understand microbial contributions to reproductive disorders and identify potential targets for precision diagnostics and personalized therapies.2
BEVs: Structure and function
Both Gram-negative and Gram-positive bacteria release BEVs that may contain proteins, nucleic acids, and metabolites capable of influencing host cells. In Gram-negative species, outer membrane vesicles (OMVs) form by blebbing the outer membrane, whereas Gram-positive bacteria release vesicles through the enzymatic degradation of their thick peptidoglycan cell wall. BEVs can cross epithelial barriers and enter host cells via endocytosis, phagocytosis, or membrane fusion, enabling delivery of cargo to recipient cells.3,4,19
Components such as lipopolysaccharide (LPS) and proteins, such as proteases, induce inflammation and promote infectivity, whereas nucleic acids, such as ribonucleic acid (RNA), enable the transfer of genetic material between cells. BEV metabolites may also regulate intercellular signaling, allowing bacteria to survive changing environmental conditions and develop resistance to treatment. BEVs also modulate immune cell differentiation and activation via pattern recognition receptors, thereby influencing downstream cytokine responses.1,3,5,19
BEVs in PCOS
PCOS is an endocrine and metabolic disorder characterized by hyperandrogenism, insulin resistance, and chronic low-grade inflammation. Increasing evidence suggests that gut dysbiosis, which is defined as an imbalance in the composition of the intestinal microbiome, contributes to the development of these metabolic and inflammatory disturbances. Alterations in the gastrointestinal microbiome can influence systemic inflammation and endocrine signaling relevant to ovarian function.2,7
In women with PCOS, gut dysbiosis often presents with altered abundances of pro-inflammatory and short-chain-fatty-acid–producing taxa. BEVs released by these microorganisms can impair insulin signaling by activating the toll-like receptor 4 (TLR4)–NF-κB pathways and upregulating cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).6,7 Consistent with this, gut-derived BEVs have been observed in human circulation and increase with age and intestinal permeability, indicating a plausible route for metabolic and inflammatory impact.11
In vivo preclinical studies have reported similar observations: fecal microbiota transplants (FMT) from women with PCOS into germ-free mice reproduce PCOS-like traits, including elevated testosterone, anovulation, weight gain, and insulin resistance. The PCOS-FMT group showed enrichment of taxa, including Phocaeicola and Oscillospiraceae, supporting a microbiota-mediated disruption of endocrine-metabolic pathways.8
In humans, circulating BEVs have the potential to improve precision diagnostics. Circulating BEV levels and composition vary with age and disease status in blood, and single-particle analyses indicate a dominant gut microbial origin.11
Sweat and serum EV profiling have identified 1,209 human and 1,594 bacterial proteins, thereby highlighting BEVs as non-invasive markers for monitoring microbial and metabolic health.9 Moreover, serum EV analysis in 88 renal cell carcinoma patients revealed three bacterial taxa, including Bacteroidia, TM7-1, and Sphingomonadales, that, when combined into a sensitive diagnostic ‘BTS index,’ correlate with treatment outcomes. This oncology example illustrates how bacteria-derived nucleic acids within EVs can serve as disease biomarkers, a principle that may be adaptable to metabolic–reproductive contexts pending validation.5,10
BEVs in serum and feces, including lipopolysaccharide and lipoteichoic acid vesicles, have also been used as biomarkers for the presence of Gram-negative and Gram-positive bacteria, respectively, with levels varying by age and intestinal permeability. BEVs have also been detected in neonatal meconium, which is typically dominated by Streptococcus and Staphylococcus, thus suggesting early-life host-microbiome communication. These early-life BEV signatures support the concept that vesicle-mediated host–microbe signaling begins perinatally.10-12
 Image Credit: Jo Panuwat D / Shutterstock.com
Image Credit: Jo Panuwat D / Shutterstock.com
BEVs in endometriosis
Endometriosis is a chronic estrogen-dependent inflammatory disorder characterized by ectopic endometrial tissue, immune dysregulation, and persistent pelvic inflammation. BEVs contribute to disease pathogenesis by transferring proteins, lipids, and nucleic acids from bacteria to host cells. Current evidence in endometriosis primarily indicates associations between dysbiosis/EV signaling and disease features; definitive causal roles for bacterial BEVs remain under active investigation.13,15,16
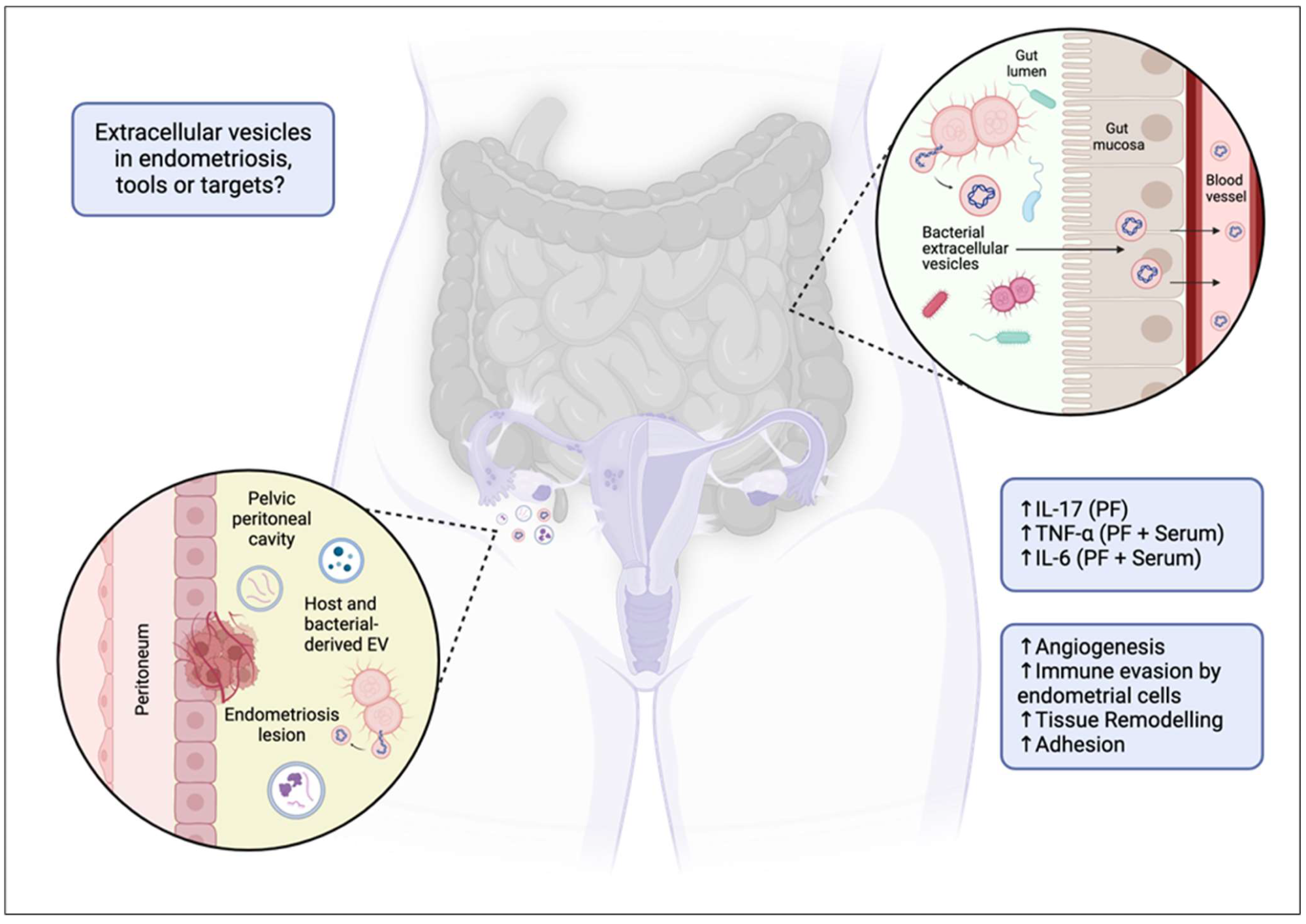
Extracellular vesicles as targets or tools in endometriosis? Proposed mechanism of extracellular vesicle (EV) and bacterial EV (BEV) involvement in endometriosis. The diagram illustrates how a dysbiotic gut may facilitate the translocation of BEVs across the gut mucosa into systemic circulation. These vesicles, along with host EVs, can migrate to the pelvic peritoneal cavity, contributing to the pathophysiology of endometriosis by promoting angiogenesis, immune evasion by endometrial cells, tissue remodelling, and adhesion. The associated increase in inflammatory markers (IL-17, TNF-α, IL-6, etc.) in the peritoneal fluid (PF) and serum is also indicated, highlighting their potential role in disease progression. ↑ = increased.13
The subsequent immune response is associated with upregulated pattern recognition signaling (e.g., TLRs) and pro-inflammatory cytokines/chemokines (e.g., IL-8). Thereafter, macrophage polarization and immune evasion prevent the clearance of ectopic tissue. BEV-triggered cytokine milieus can also affect stromal–immune crosstalk that supports lesion survival.13,14,16
BEVs also promote lesion development by increasing myeloid-derived suppressor cells (MDSCs), which enhances angiogenesis and vascular remodeling. Evidence from other diseases supports this twofold role, as BEVs from pathogens can induce endothelial chemokines and IL-8.13
Dysbiosis and compromised intestinal permeability may permit the entry of BEVs into the peritoneal cavity, leading to the emergence of additional lesions. Several studies have reported altered microbial profiles in patients with endometriosis. Peritoneal and genital tract microbiome shifts are being explored alongside EV cargo to refine non-invasive diagnostic strategies.14,15, 17
EV-derived small RNAs from serum and vaginal samples are being investigated as diagnostic markers in endometriosis. Non-invasive BEV-based diagnostics could reduce the need for surgical procedures, enabling earlier intervention and improving the quality of life for women with endometriosis.16,17
Bacterial extracellular vesicles repress RNase1 in human lung endothelial cells
Clinical potential of BEVs
BEVs in blood, urine, saliva, and vaginal secretions carry disease- and species-specific molecular signatures, thereby enabling liquid biopsy approaches for early and non-invasive detection. Because BEVs traverse biological barriers (e.g., the gut–blood barrier), their cargo can reflect both local microbial states and systemic host responses.3,11,19
The composition of BEVs can also reflect host metabolic or immune states, which may offer dynamic biomarkers of disease progression and treatment response. In fact, previous studies on cancer, inflammatory, and infectious diseases suggest that BEV constituents can indicate disease presence, progression, and therapeutic efficacy.5 Standardized isolation and characterization are prerequisites for translating BEV biomarkers to clinical assays.20
Nano-sized, stable, and naturally biocompatible, BEVs can travel long distances, cross biological barriers, and merge with cell membranes while protecting drugs from degradation, thus making them ideal vehicles for drug delivery. Engineered BEVs can deliver small molecules, proteins, or gene-editing tools, such as small interfering RNA (siRNA) or clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9). Surface modifications with ligands or antibodies can further enhance precise delivery and minimize off-target effects. Emerging therapeutic concepts include probiotic- or gut-derived BEVs to attenuate inflammation and modulate metabolism.7,19
In personalized medicine, BEVs offer the potential to combine diagnostic and therapeutic applications. Profiling patient-specific BEVs may guide individualized interventions based on microbial composition, metabolic status, or immune profile.
BEVs can also be combined with conventional treatments to improve drug efficacy and reduce side effects through microbiome-informed targeting. Gut-derived or probiotic BEVs may also provide postbiotic benefits, restoring host-microbial balance. Nonetheless, regulatory guidance specific to EV/BEV therapeutics is still evolving, highlighting the need for robust quality, safety, and nonclinical evaluation frameworks.20
Research frontiers and limitations
Despite the promise of biomedical applications, significant challenges remain in translating BEV research from animal models to humans, due to species differences in immunity, microbiota, and disease complexity that limit predictive accuracy. Moreover, immune recognition of BEV-associated molecular patterns (PAMPs) can provoke species-specific inflammatory responses, thereby affecting safety and efficacy.19
Technical challenges in isolating and characterizing BEVs similarly limit progress. Current methods, such as ultracentrifugation, size-exclusion chromatography, and immunoaffinity separation, are labor-intensive and variable, and may cause vesicular damage.
Heterogeneity in BEV composition and contamination from host-derived EVs further complicate reproducibility. Thus, standardized protocols and transparent reporting are essential to ensure consistent results. Harmonized reporting, following community guidelines and rigorous negative controls, is particularly important for low-biomass samples.14,20
Ethical and regulatory considerations also constrain clinical applications, as BEVs from pathogenic bacteria may carry toxins. Engineered BEVs also increase biosecurity concerns regarding gene transfer and long-term effects.
Long-term safety data are limited, and regulatory frameworks for BEV-based therapies remain underdeveloped. Ongoing technological advances are supporting the development of risk assessment, quality control, and regulatory frameworks to support clinical translation.19,20
Conclusions
BEVs carry diverse molecular cargo, including nucleic acids, lipids, and functional proteins, which can serve as non-invasive biomarkers for early diagnosis and monitoring of PCOS and endometriosis. Engineered BEVs also offer promising strategies for developing targeted delivery systems to modulate disease pathways and promote tissue repair.
References
- Jeong, G., Khan, F., Tabassum, N., et al. (2024). Bacterial extracellular vesicles: Modulation of biofilm and virulence properties. Acta Biomaterialia 178; 13-23. DOI:10.1016/j.actbio.2024.02.029, https://www.sciencedirect.com/science/article/abs/pii/S1742706124001004.
- García-Velasco, J. A., Budding, D., Campe, H., et al. (2020). The reproductive microbiome – clinical practice recommendations for fertility specialists. Reproductive BioMedicine Online 41(3); 443-453. DOI:10.1016/j.rbmo.2020.06.014, https://www.sciencedirect.com/science/article/pii/S1472648320303394.
- Ñahui Palomino, R. A., Vanpouille, C., Costantini, P. E., & Margolis, L. (2021). Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathogens 17(5); e1009508. DOI:10.1371/journal.ppat.1009508, https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009508.
- Rima, M., Dakramanji, M., El Hayek, E., et al. (2025). Unveiling the wonders of bacteria-derived extracellular vesicles: From fundamental functions to beneficial applications. Heliyon 11(4). DOI:10.1016/j.heliyon.2025.e42509, https://www.sciencedirect.com/science/article/pii/S2405844025008898.
- Bhanu, P., Godwin, A. K., Umar, S., & Mahoney, D. E. (2025). Bacterial Extracellular Vesicles in Oncology: Molecular Mechanisms and Future Clinical Applications. Cancers 17(11); 1774. DOI:10.3390/cancers17111774, https://www.mdpi.com/2072-6694/17/11/1774.
- Kushawaha, B., Rem, T. T., & Pelosi, E. (2025). Harnessing Microbiome, Bacterial Extracellular Vesicle, and Artificial Intelligence for Polycystic Ovary Syndrome Diagnosis and Management. Biomolecules 15(6); 834. DOI:10.3390/biom15060834, https://www.mdpi.com/2218-273X/15/6/834.
- Yang, L., Liu, T., Liao, Y., et al. (2024). Potential therapeutic application and mechanism of gut microbiota-derived extracellular vesicles in polycystic ovary syndrome. Biomedicine & Pharmacotherapy 180. DOI:10.1016/j.biopha.2024.117504, https://www.sciencedirect.com/science/article/pii/S0753332224013908.
- Huang, F., Deng, Y., Zhou, M., et al. (2024). Fecal microbiota transplantation from patients with polycystic ovary syndrome induces metabolic disorders and ovarian dysfunction in germ-free mice. BMC Microbiology 24. DOI:10.1186/s12866-024-03513-z, https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-024-03513-z.
- Zhyvolozhnyi, A., Samoylenko, A., Bart, G., et al. (2024). Enrichment of sweat-derived extracellular vesicles of human and bacterial origin for biomarker identification. Nanotheranostics 8(1);48-63. DOI:10.7150/ntno.87822, https://www.ntno.org/v08p0048.htm.
- Uemura, T., Kawashima, A., Jingushi, K., et al. (2023). Bacteria-derived DNA in serum extracellular vesicles are biomarkers for renal cell carcinoma. Heliyon 9(9). DOI:10.1016/j.heliyon.2023.e19800, https://www.sciencedirect.com/science/article/pii/S2405844023070081.
- Ou, Z., Huang, X., Xue, Y., et al. (2023). Single‐particle analysis of circulating bacterial extracellular vesicles reveals their biogenesis, changes in blood, and links to the intestinal barrier. Journal of Extracellular Vesicles 12(12). DOI:10.1002/jev2.12395, https://onlinelibrary.wiley.com/doi/10.1002/jev2.12395.
- Turunen, J., Tejesvi, M. V., Suokas, M., et al. (2023). Bacterial extracellular vesicles in the microbiome of first-pass meconium in newborn infants. Pediatric Research 93(4); 887-896. DOI:10.1038/s41390-022-02242-1, https://www.nature.com/articles/s41390-022-02242-1.
- Wagner, M., Hicks, C., & Combes, V. (2024). The Critical Role of Host and Bacterial Extracellular Vesicles in Endometriosis. Biomedicines 12(11). DOI:10.3390/biomedicines12112585, https://www.mdpi.com/2227-9059/12/11/2585.
- Duval, C., Wyse, B. A., Tsang, B. K., & Librach, C. L. (2024). Extracellular vesicles and their content in the context of polycystic ovarian syndrome and endometriosis: a review. J Ovarian Research 17(1). DOI:10.1186/s13048-024-01480-7, https://ovarianresearch.biomedcentral.com/articles/10.1186/s13048-024-01480-7.
- Uzuner, C., Mak, J., & Condous, G. (2023). The bidirectional relationship between endometriosis and microbiome. Frontiers in Endocrinology 14. DOI:10.3389/fendo.2023.1110824, https://www.frontiersin.org/articles/10.3389/fendo.2023.1110824.
- Chu, X., Hou, M., Li, Y., et al. (2024). Extracellular vesicles in endometriosis: Role and potential. Frontiers in Endocrinology 15. DOI:10.3389/fendo.2024.1365327, https://www.frontiersin.org/articles/10.3389/fendo.2024.1365327.
- Kovács, Z., Glover, L., Reidy, F., et al. (2021). Novel diagnostic options for endometriosis – Based on the glycome and microbiome. Journal of Advanced Research 33; 167-181. DOI:10.1016/j.jare.2021.01.015, https://www.sciencedirect.com/science/article/pii/S2090123221000229?via%3Dihub.
- Moghaddam, Z. S., Dehghan, A., Halimi, S., et al. (2025). Bacterial Extracellular Vesicles: Bridging Pathogen Biology and Therapeutic Innovation. Acta Biomaterialia 200; 1-20. DOI:10.1016/j.actbio.2025.05.028, https://www.sciencedirect.com/science/article/pii/S1742706125003526.
- Wang, Y., Zu, M., Li, B., et al. (2025). Application of bacterial extracellular vesicles in gastrointestinal diseases. Trends in Biotechnology. DOI:10.1016/j.tibtech.2025.05.022, https://www.sciencedirect.com/science/article/abs/pii/S0167779925002082.
- Xu, G., Jin, J., Fu, Z., et al. (2025). Extracellular vesicle-based drug overview: Research landscape, quality control, and nonclinical evaluation strategies. Signal Transduction and Targeted Therapy 10(1); 1-26. DOI:10.1038/s41392-025-02312-w, https://www.nature.com/articles/s41392-025-02312-w.
Further Reading
Last Updated: Oct 29, 2025