This article and associated images are based on a poster originally authored by Celia Garcia, Carl Peters and Supriya Singh and presented at ELRIG Drug Discovery 2025 in affiliation with BMG LABTECH and Cell Signaling Technology.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Tau was originally identified for its role as a microtubule associated protein. It has since been shown to be important in axonal growth and function due to its influence on neuronal microtubule stability. The function of tau is regulated by post-translational modification at more than 50 sites. Altered amount of phospho-tau is included in these regulatory modifications (Figure 1).
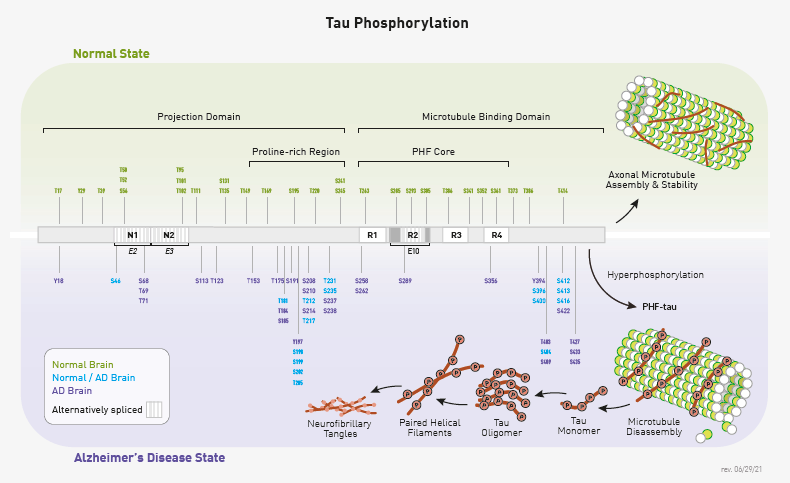
Fig. 1. Comparison of phospho-tau modifications in normal and Alzheimer’s disease states phospho-tau modifications have been mapped to the multiple locations shown here. Image Credit: Cell Signaling Technology, Inc. Used with permission.
Tau is of significant clinical and pharmacological research interest because it is among the main components in the neurofibrillary tangles characteristic of Alzheimer’s disease (AD).1 Subsequently, it was discovered that high levels of phospho-tau are also present in the disease state (Figure 1). The precise relationship between tau phosphorylation, tau aggregation, and disease progression is still under investigation.
Here, the team show the results of detection, by ELISA, of two phospho-tau modifications at threonine 181 and 217. Both have been proposed as functional biomarkers to discriminate between the healthy state and AD.2 Studies with tau mutations that mimic phospho-tau at Thr217 have shown that it increases phospho-tau at additional sites and increases fibrilization.3
Materials & methods
- Novus Biologicals: Human Adult Whole Alzheimer’s Brain Tissue Lysate (NB820-59363) and Human Adult Whole Normal Brain Tissue Lysate (NB820-59177)
- FastScanTM Phospho-Tau (Thr181) ELISA Kit #58537 and PathScan® Phospho-Tau (Thr217) Chemiluminescent Sandwich ELISA Kit #87749 (Cell Signaling Technology)
- CLARIOstarPlus
Experimental procedure
- Lysates provided at a five mg/mL concentration were diluted in the included FastScan or PathScan buffer.
- A 12-point two-fold dilution series was prepared for each lysate. For FastScan, the starting lysate concentration is 0.5 mg/ml, and 50 μL was used per well. For PathScan, the starting concentration is one mg/mL and 25 μL was used per well.
- ELISA experiments were carried out according to the kit instructions found here:
https://cst-science.com/58537-protocol
https://cst-science.com/87749-protocol
Instrument settings for PathScan Phospho-Tau (Thr217) Chemiluminescent Sandwich ELISA Kit #87749. Source: ELRIG (UK) Ltd.
| . |
. |
. |
| Optic settings |
Luminescence |
| Emission |
No filter |
| Gain |
EDR |
| Focal height |
11.0 mm |
| General settings |
Optic used |
Top |
| Meas. Int. time |
1.00 s |
Instrument settings for FastScan Phospho-Tau (Thr181) ELISA Kit #58537. Source: ELRIG (UK) Ltd.
| . |
. |
. |
| Optic settings |
Absorbance Spectrum |
| Wavelength range |
400-700 nm |
| Step width |
Two nm |
| General settings |
Flashes / well |
100 |
| Settling time |
0.5 s |
Results & discussion
FastScan Phospho-Tau (Thr181) ELISA Kit #58537 results are achieved with a single washing step, as the use of a single tagged antibody allows the entire antibody and analyte complex to be immobilized in the well. In the detection step, collecting spectral data is useful to troubleshoot any unexpected results.
The results shown in Figure 2 support that the phospho-tau at Thr181 can be used to discriminate between normal and AD brain tissue samples.
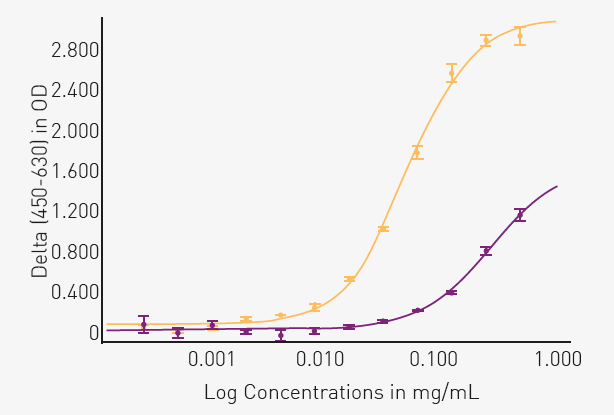
Fig. 2. Comparing phospho-tau (Thr 181) levels in normal and AD brain tissue. Image Credit: Image courtesy of Celia Garcia et al., in partnership with ELRIG (UK) Ltd.
Absorbance measurements at 630 nm were subtracted from those at 450 nm. The result was plotted against the protein concentration of the tissue samples from normal (purple) and AD (yellow) brains. A four-parameter fit curve conforms well to the data (R2 = 0.99 for both tissues).
The team next tested the PathScan Phospho-Tau (Thr217) Chemiluminescent Sandwich ELISA Kit #87749. This kit can be employed with a smaller sample size due to the increase in signal and sensitivity possible with a luminescent assay.
Figure 3 shows a high dynamic range of luminescent signal, which is especially evident in high concentration samples of AD tissue.
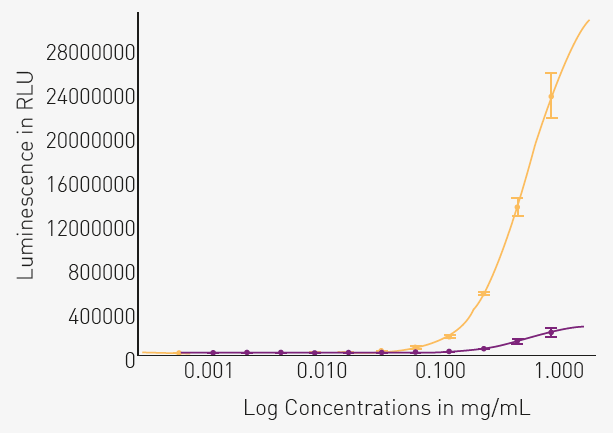
Fig. 3. Comparing phospho-tau (Thr217) levels in normal and AD brain tissue. Image Credit: Image courtesy of Celia Garcia et al., in partnership with ELRIG (UK) Ltd.
Luminescence was plotted against the protein concentration of the tissue samples from normal (purple) and AD (yellow) brains. A four-parameter fit curve conforms well to the data (R2 = 0.97 or higher for both tissues).
Conclusion
Both the FastScan Phospho-Tau (Thr181) ELISA Kit #58537 and PathScan Phospho-Tau (Thr217) Chemiluminescent Sandwich ELISA Kit #87749 from Cell Signaling Technology generate the expected results in control and experimental human brain tissue samples.
In agreement with previously reported literature, both tau phosphorylation sites functionally distinguish normal from AD tissue. Thr217 appears to be somewhat superior to Thr181 as a potential biomarker for AD.
CLARIOstar technologies are advantageous for detecting spectral absorbance data and high dynamic range luminescence, ensuring optimal performance of these ELISA kits.
References
- Wegmann, S., Biernat, J. and Mandelkow, E. (2021). A current view on Tau protein phosphorylation in Alzheimer’s disease. Current Opinion in Neurobiology, [online] 69, pp.131–138. https://doi.org/10.1016/j.conb.2021.03.003.
- Thijssen, E.H., et al. (2021). Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. The Lancet Neurology, [online] 20(9), pp.739–752. https://doi.org/10.1016/s1474-4422(21)00214-3.
- Wang, X., et al. (2021). T217-Phosphorylation Exacerbates Tau Pathologies and Tau-Induced Cognitive Impairment. Journal of Alzheimer’s Disease, pp.1–16. https://doi.org/10.3233/jad-210297.
About BMG LABECH
BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025