The National Institute for Health and Care Excellence (NICE) has developed a Medtech Innovation Briefing (MIB) on Zilico Ltd’s cervical cancer diagnostic system, ZedScan.
NICE first developed the briefings in 2013 to help boost and speed-up the uptake of innovative and promising technologies which have the potential to improve patient health and increase NHS productivity.
Medtech Innovation Briefings provide a description of a medical technology, including its likely place in therapy, the costs of using the technology and a critical review of the strengths and weaknesses of the relevant published evidence.
The purpose of the MIB is to provide objective information on device and diagnostic technologies to aid local decision-making by clinicians, managers and procurement professionals. By making this information available, NICE helps to avoid the need for NHS organisations to produce similar information for local use.
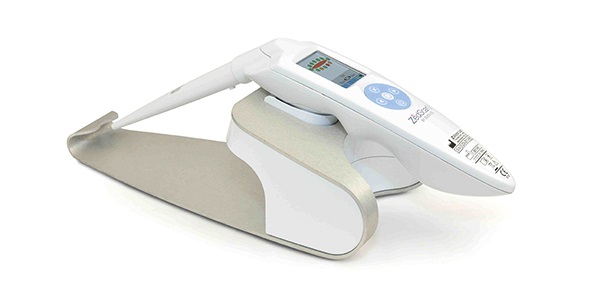 Zilico Ltd’s cervical cancer diagnostic system, ZedScan
Zilico Ltd’s cervical cancer diagnostic system, ZedScan
The NICE MIB has been developed on ZedScan as an adjunct to colposcopy in women with suspected cervical intra-epithelial neoplasia.
The ZedScan system is used alongside colposcopy on women identified with an abnormal smear result.
ZedScan offers clinical benefits by identifying those patients who require treatment at first visit, reducing the number of cervical biopsies performed on each patient by pin-pointing the optimum site for biopsy and reducing follow-up appointments - benefiting the patient and the hospital.
These benefits translate into better health economics as identified in an independent report by the School of Health and Related Research (ScHARR).
The MIB states that this economic evaluation is a “well executed document with a clear statement of limitations and assumptions.” Pg 28, NICE Medtech Innovation Briefing 20 (2015)
The NICE MIB for ZedScan identifies three key benefits from the report:
“For both the 'see and treat' and 'triage' clinics, the model showed a reduction in overtreatment, adverse events and related treatment costs.
“Lower biopsy rates were associated with the ZedScan, with 1–1.02 biopsies per woman having colposcopy with the ZedScan compared with 1.72 with standard care, and this reduced related costs.
“A cost saving was also illustrated from the reduction in follow-up colposcopy appointments over the timeframe of the model.”
Pg 26 - 27, NICE Medtech Innovation Briefing 20 (2015)
ZedScan is a portable and non-visual/non-optical system. The MIB also states that “NICE is not aware of any other CE Marked devices that have a similar function to the ZedScan”.
Pg 8, NICE Medtech Innovation Briefing 20 (2015)
The NICE MIB follows the publication of a peer-reviewed multi-centre study in BJOG: An International Journal of Obstetrics & Gynaecology, and the health economics evaluation and a real-clinic case study on over 500 patients. .
Click here to read the full NICE MIB.