May 24 2016
HIV, the virus that causes AIDS, has become one of the world’s most serious health and development challenges. Currently, there are approximately 36.9 million people living with HIV and tens of millions of people have died of AIDS-related causes since the beginning of the epidemic in 1981. HIV not only affects the health of individuals, it impacts households, communities, and the development and economic growth of nations – there is still no cure.
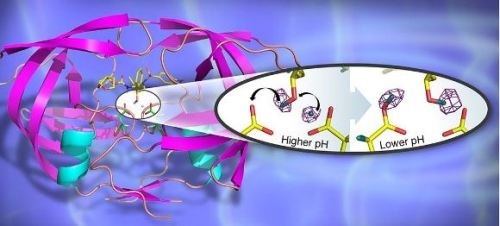
A 3D structure of the HIV-1 protease in cartoon representation with bound clinical drug darunavir (shown as sticks). The catalytic site contains two closely positioned aspartic acid residues. The insert depicts the hydrogen transfer reaction in the catalytic site, captured for the first time by neutron crystallography. (Credit: Jill Hemman and Andrey Kovalevsky, Oak Ridge National Laboratory)
HIV treatment includes medications to prevent and treat the many opportunistic infections that can occur when the immune system is compromised by HIV, as well as the use of antiretroviral therapy (ART) to attack the virus itself, with the aim of halting the development of AIDS. ART, first introduced in 1996, has led to dramatic reductions in morbidity and mortality; globally, 40% of people living with HIV are receiving treatment. HIV-1 protease is an enzyme responsible for maturation of virus particles into infectious HIV virions, which ultimately leads to the development of AIDS. Without effective HIV-1 protease activity, HIV virions remain non-infectious – with this integral role in HIV replication, the disruption of HIV-1 protease activity is therefore a key target for successful ART drugs.
The design of effective ART drugs has been led by the structures of HIV-1 protease/drug complexes determined using X-ray crystallography, and although this has led to the development of commercially available drugs, a limitation of the method is that the positions of mobile hydrogen atoms and protons cannot be determined using X-rays, and yet knowledge of their location and movement is vital for guiding the design of more effective drug therapies since hydrogen-bonding interactions play a key role in how effective a drug binds to its target.
Recently however, a collaboration between Georgia State University, USA, Oak Ridge National Laboratory, USA (ORNL), and the Institut Laue-Langevin, France (ILL) has used neutron crystallography to probe the structure of HIV-1 protease in complex with the clinical inhibitor darunavir, allowing details of the hydrogen-bonding interactions in the active site to be determined and revealing ways to enhance drug-binding and reduce drug-resistance. The group was also able to shed light on the sensitivity to pH of the enzyme’s catalytic activity.
By determining structures at different pHs, the group was able to directly observe the positions of hydrogen atoms before and after a pH-induced two-proton transfer between the drug and enzyme. The low-pH proton configuration in the catalytic site, critical for the catalytic action of this enzyme, was shown to be triggered by electrostatic effects arising from protonation state changes of surface residues far from the active site. These details can help assist in the design of new more effective ART drugs and were only possible through the use of neutron crystallography.
ILL Instrument Scientist Dr Matthew Blakeley said:
These results highlight that neutrons represent a superb probe to obtain structural details for proton transfer reactions in biological systems.
R&D Scientist at the ORNL, Dr Andrey Kovalevsky added: “Darunavir’s structure allows it to create more hydrogen bonds with the protease active site than most drugs of its type, while the backbone of HIV-1 protease maintains its spatial conformation in the presence of mutations, meaning Darunavir-protease interaction is less likely to be disrupted by a mutation. Given these characteristics, Darunavir is an excellent therapy target to refine and therefore enhance HIV treatment.” In fact, in the US and UK, healthcare costs were estimated to be lower with Darunavir than other similar drugs, understandably making Darunavir a key focus for drug innovation in the HIV therapy area.
Direct observation of proton transfer in chemical and biological systems is challenging; macromolecular neutron crystallography has been pivotal in providing key details regarding hydrogen that were required in order to answer long-standing questions about the enzyme mechanism of this important HIV drug target. Moreover, the observation that changes in amino-acid protonation-states distant from the active-site can trigger a change in hydrogen configuration in the active-site may apply to other aspartic proteases, and perhaps enzymes more generally. With the recent improvements that have been made, the field of macromolecular neutron crystallography is expanding, with studies addressing a variety of important biological processes from protein-folding to antibiotic resistance and proton transport across biological membranes.