Leica Microsystems is the first company in the US receiving FDA 510(k) clearance for the visualization of cerebrovascular blood flow in conjunction with fluorescein.
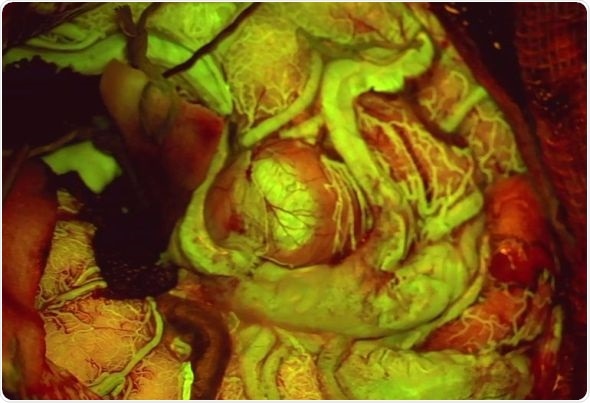
View of an Arteriovenous Malformation (AVM) viewed with FL560 fluorescence. Photo courtesy of Cleopatra Charalampaki, MD, PhD, Professor of Neurosurgery, Department of Neurosurgery, Cologne Medical Center, Germany
Leica Microsystems is proud to be the first company to attain FDA 510(k) clearance for its FL560 fluorescence microscope filter for visualization of cerebrovascular blood flow in conjunction with the dye fluorescein. When integrated into a M530 OH6 neurosurgical microscope, FL560 fluorescence provides real-time, high-contrast visualization of both cerebral anatomy in native color and fluorescent blood flow. With this combined view, the surgeon has more information to aid assessment and decision-making during vascular neurosurgery.
Assessing cerebral anatomy and vascular flow, particularly in smaller vessels and the areas they perfuse, can be challenging under white light or with traditional near-infrared fluorescence which only provides a black and white image. By integrating the FL560 fluorescence filter into the M530 OH6 neurosurgical microscope, the surgeon is able to view anatomical structures in white-light and fluorescent blood flow simultaneously in the oculars. This is achieved by combining premium microscope optics and illumination with a sophisticated, proprietary fluorescence filter design that effectively separates fluorescence excitation light and the observation spectrum. The result is a high-contrast image where anatomy is clearly visible and even the smallest vessels delineated.
“Leica Microsystems has been a leader in advanced surgical visualization for decades. We have achieved a number of “first’s” over the years including the first FDA 510(k) clearance of a microscope for intra-operative angiography (FL800) and the first company offering a surgical microscope to integrate three types of fluorescence capabilities (TriFluoro). I am proud that our team again was able to be the innovation leader by being the first company to achieve FDA clearance for cerebrovascular fluorescence imaging (FL560). This demonstrates our commitment to deliver clinically relevant and validated solutions to clinicians that allow them to make crucial surgical decisions and as a result improve patient outcomes,” says Markus Lusser, President Leica Microsystems.
FL560 fluorescence can aid the surgeon’s visualization and thus intrasurgical decisions in a variety of neurovascular cases including Arteriovenous Malformations (AVM) and aneurysms.
FL560 fluorescence from Leica Microsystems has been designed to allow full integration into existing or new M530 OH6 neurosurgical microscopes at any time. The M530 OH6 microscope was the first to feature TriFluoro technology. This enables FL560 and two other types of fluorescence to be installed into a single microscope. Full integration also facilitates a smooth workflow with one-touch activation of different fluorescence modes via microscope handgrip or wireless footswitch, further supporting the surgical workflow.