Proteins inside a cell are often made at the site where they are used, rather than being shipped long distances. This is so with nerve cells which have long cell extensions in the form of axons and dendrites, up to four feet long in some cases. However, for this to happen, RNA has to travel from the nucleus (where it is produced) to outlying parts of the cell. A new study shows that RNA hitches rides on moving lysosomes, using a tethering protein called annexin 11, to get to where it needs.
When this doesn’t happen, the consequences may be catastrophic, as in amyotrophic lateral sclerosis (ALS). This rare but fatal disease causes the nerves to break down progressively, leading to paralysis and then death. The annexin 11 gene (ANXA11) has been blamed for a familial type of ALS. Mutations in this gene act in different ways, but ultimately all of them seem to prevent the normal transport of RNA within neurons to keep things running smoothly, producing proteins as and where needed.
Single strand ribonucleic acid, RNA research and therapy, 3d illustration Credit: nobeastsofierce / Shutterstock
Why RNA needs to hitch-hike
The problem with RNA is that it is not contained in neat membrane-bound packages, unlike mitochondria, which could be shifted by tiny cell motors, proteins called kinesin or dynein, which operate via microtubules. Instead, RNA binds to RNA-binding proteins (RBP) to form RNA granules. These structures can travel within axons and dendrites, the cell extensions from the neuronal cell body. This also involves a microtubule-motor apparatus. However, the mechanism by which the RNA is tied down on this ‘cargo carrier’ has been described in the current study for the first time.
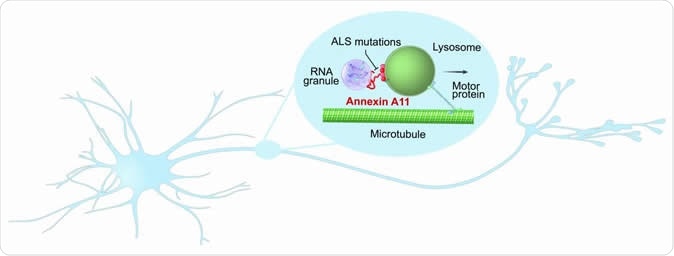
Researchers discovered that annexin A11, a gene linked to a rare form of ALS, may play a critical role in the transport of important, RNA encoded housekeeping instructions throughout neurons. Their results suggest that the gene does this by hitching RNA granules onto traveling lysosomes and that disease-causing mutations prevent hitchhiking. Image Credit: NIH/NINDS
Many neurological diseases such as ALS and frontotemporal dementia (FTD) are due to mutations in microtubule or motor components. However, these conditions also show mutations in lysosome-related genes. Lysosomes are sac-like organelles within the cell that are best known for gobbling up waste residues inside the cell to clean it up. However, they are also attached to the cell motor proteins through adapter proteins, and are observed to travel over long distances within neuronal axons and dendrites.
This leads to another interesting way in which the cell moves around cargo: by allowing them to hitch-hike on cargo that is already bound long-distance, rather than attaching them directly to the microtubules or motors. This is how RNA granules grab a ride on endosomes inside fungal cells.
The current study confirms that this also happens in mammalian cells, with RNA granules hitching a ride with lysosomes. The researchers also found that ANXA11, the protein linked to ALS, is the dynamic coupler that holds the RNA granule to the lysosomes. Thus the mutations in this gene prevent this coupling, blocking the transport of RNA granules within the neurons. This study refers to all vesicles within the cell as lysosomes, but actually, as the researchers clarify, they mean all cells which are positive for the lysosome marker LAMP-1, including endosomes and inactive sacs.
The study shows the lysosome to be at the heart of RNA trafficking in the nucleus, describes the facilitating role of ANXA11 in docking the RNA granule on the lysosome without the help of membranes, and throws light on how RNA transport is different.
Fascinating details
When researchers looked at the ability of RNA granules to move with lysosomes along microtubules over long distances within axons, they observed that the lysosomes don’t engulf the RNA granules but act as a vehicle for transport. The tether connecting the two is the ANXA11 protein. This protein has separate regions to bind both to lipid membranes like the lysosomal membrane, and to phase-separated RNA granules through its phase-separating properties. These occur at the two ends of the ANXA11 molecule, the nitrogen-terminal and the carbon-terminal ends respectively.
For this coupling to occur, both calcium and phospholipid residues are needed.
Multiple mutations have been observed in the ANXA11 gene in ALS. Mutations at the C- and N-terminal ends cause abnormally firm connections to take place between this protein and the RNA granules, which affects their phase transition. As a result, the RNA granules irreversibly form a gel instead of being a liquid-liquid phase that can transition to a gel-like state and back again.
Some ANXA11 mutations reduce the interaction between this protein and the phase-separated RNA granules, altering the properties of the granules. C-terminal mutations also reduce the affinity of ANXA11 to phospholipid membranes, preventing its binding to lysosomes, and thus preventing RNA transport.
Conclusion
The study thus confirmed that ANXA11 tethers RNA granules to lysosomes, allowing the granules within the axons to hitch-hike on these organelles and reach distant locations within the neuron. Mutations which prevent this interaction also prevent this hitch-hiking, thus impairing the transport of axonal RNA granules and their delivery to other parts of the cell for protein translation. The most harmful of these mutations were those at the C-terminal end because they caused the loss of phase-separation and the ability to interact with a phospholipid membrane, thus leading to a severe loss of ANXA11.
Thus ALS is due to mutations that cause even a relatively small loss of RNA transport efficiency, which could result in a large-scale interruption of neuronal metabolism and the breakdown of synaptic transmission. Thus dysfunctional RNA transport could be the final common pathway through which multiple mutations act to disrupt the interaction between lysosomes and RNA granules.
The study was published in the journal Cell on September 19, 2019.
Journal reference:
RNA granules hitchhike on lysosomes for long-distance transport, using annexin a11 as a molecular tether. Ya-Cheng Liao, Michael S. Fernandopulle, Guozhen Wang, Heejun Choi, Ling Hao, Catherine M. Drerup, Rajan Patel, Seema Qamar, Jonathon Nixon-Abell, Yi Shen, William Meadows, Michele Vendruscolo, Tuomas P.J. Knowles, Matthew Nelson, Magdalena A. Czekalska, Greta Musteikyte, Mariam A. Gachechiladze, Christina A. Stephens, H. Amalia Pasolli, Lucy R. Forrest, Peter St George-Hyslop, Jennifer Lippincott-Schwartz & Michael E. Ward. Cell; Volume 179, Issue 1, P147-164.e20, September 19, 2019. https://doi.org/10.1016/j.cell.2019.08.050. https://www.cell.com/cell/fulltext/S0092-8674(19)30969-9